Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes
- PMID: 20090940
- PMCID: PMC2806905
- DOI: 10.1371/journal.pone.0008732
Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes
Abstract
Background: Deoxygenation of sickle erythrocytes activates a cation permeability of unknown molecular identity (Psickle), leading to elevated intracellular [Ca(2+)] ([Ca(2+)](i)) and subsequent activation of K(Ca) 3.1. The resulting erythrocyte volume decrease elevates intracellular hemoglobin S (HbSS) concentration, accelerates deoxygenation-induced HbSS polymerization, and increases the likelihood of cell sickling. Deoxygenation-induced currents sharing some properties of Psickle have been recorded from sickle erythrocytes in whole cell configuration.
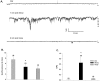
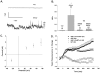
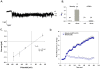
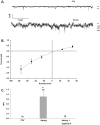
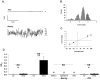
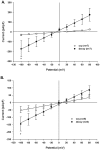
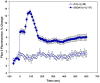
Methodology/principal findings: We now show by cell-attached and nystatin-permeabilized patch clamp recording from sickle erythrocytes of mouse and human that deoxygenation reversibly activates a Ca(2+)- and cation-permeable conductance sensitive to inhibition by Grammastola spatulata mechanotoxin-4 (GsMTx-4; 1 microM), dipyridamole (100 microM), DIDS (100 microM), and carbon monoxide (25 ppm pretreatment). Deoxygenation also elevates sickle erythrocyte [Ca(2+)](i), in a manner similarly inhibited by GsMTx-4 and by carbon monoxide. Normal human and mouse erythrocytes do not exhibit these responses to deoxygenation. Deoxygenation-induced elevation of [Ca(2+)](i) in mouse sickle erythrocytes did not require KCa3.1 activity.
Conclusions/significance: The electrophysiological and fluorimetric data provide compelling evidence in sickle erythrocytes of mouse and human for a deoxygenation-induced, reversible, Ca(2+)-permeable cation conductance blocked by inhibition of HbSS polymerization and by an inhibitor of strctch-activated cation channels. This cation permeability pathway is likely an important source of intracellular Ca(2+) for pathologic activation of KCa3.1 in sickle erythrocytes. Blockade of this pathway represents a novel therapeutic approach for treatment of sickle disease.
Conflict of interest statement
Figures

Similar articles
-
The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors.J Physiol. 2012 May 1;590(9):2095-105. doi: 10.1113/jphysiol.2012.229609. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411011 Free PMC article.
-
Purinergic signaling is essential for full Psickle activation by hypoxia and by normoxic acid pH in mature human sickle red cells and in vitro-differentiated cultured human sickle reticulocytes.Pflugers Arch. 2022 May;474(5):553-565. doi: 10.1007/s00424-022-02665-z. Epub 2022 Feb 16. Pflugers Arch. 2022. PMID: 35169901
-
Deoxygenation-induced cation fluxes in sickle cells: II. Inhibition by stilbene disulfonates.Blood. 1990 Jul 1;76(1):212-20. Blood. 1990. PMID: 2364172
-
The effect of abnormal hemoglobins on the membrane regulation of cell hydration.Tex Rep Biol Med. 1980-1981;40:417-29. Tex Rep Biol Med. 1980. PMID: 7034277 Review.
-
Membrane transport in sickle cell disease.Blood Cells Mol Dis. 2002 May-Jun;28(3):303-14. doi: 10.1006/bcmd.2002.0515. Blood Cells Mol Dis. 2002. PMID: 12367577 Review.
Cited by
-
The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors.J Physiol. 2012 May 1;590(9):2095-105. doi: 10.1113/jphysiol.2012.229609. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411011 Free PMC article.
-
N-ethylmaleimide activates a Cl(-)-independent component of K(+) flux in mouse erythrocytes.Blood Cells Mol Dis. 2013 Jun;51(1):9-16. doi: 10.1016/j.bcmd.2013.02.004. Epub 2013 Mar 6. Blood Cells Mol Dis. 2013. PMID: 23481459 Free PMC article.
-
Calcium in red blood cells-a perilous balance.Int J Mol Sci. 2013 May 8;14(5):9848-72. doi: 10.3390/ijms14059848. Int J Mol Sci. 2013. PMID: 23698771 Free PMC article. Review.
-
The brief life-story of irreversibly sickled cells.Biophys J. 2025 Apr 15;124(8):1179-1182. doi: 10.1016/j.bpj.2025.03.003. Epub 2025 Mar 10. Biophys J. 2025. PMID: 40070119 No abstract available.
-
On the Mechanism of Human Red Blood Cell Longevity: Roles of Calcium, the Sodium Pump, PIEZO1, and Gardos Channels.Front Physiol. 2017 Dec 12;8:977. doi: 10.3389/fphys.2017.00977. eCollection 2017. Front Physiol. 2017. PMID: 29311949 Free PMC article. Review.
References
-
- Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358:1362–1369. - PubMed
-
- Steinberg MH. Pathophysiologically based drug treatment of sickle cell disease. Trends Pharmacol Sci. 2006;27:204–210. - PubMed
-
- Steinberg MH, Brugnara C. Pathophysiological-based approaches to treatment of sickle cell disease. Annu Rev Med. 2003;54:89–112. - PubMed
-
- Brugnara C, De Franceschi L, Bennekou P, Alper SL, Christophersen P. Novel therapies for prevention of erythrocyte dehydration in sickle cell anemia. Drug News Perspect. 2001;14:208–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

