Symbiont interactions in a tripartite mutualism: exploring the presence and impact of antagonism between two fungus-growing ant mutualists
- PMID: 20090958
- PMCID: PMC2806923
- DOI: 10.1371/journal.pone.0008748
Symbiont interactions in a tripartite mutualism: exploring the presence and impact of antagonism between two fungus-growing ant mutualists
Abstract
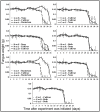
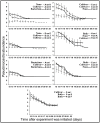
Mutualistic associations are shaped by the interplay of cooperation and conflict among the partners involved, and it is becoming increasingly clear that within many mutualisms multiple partners simultaneously engage in beneficial interactions. Consequently, a more complete understanding of the dynamics within multipartite mutualism communities is essential for understanding the origin, specificity, and stability of mutualisms. Fungus-growing ants cultivate fungi for food and maintain antibiotic-producing Pseudonocardia actinobacteria on their cuticle that help defend the cultivar fungus from specialized parasites. Within both ant-fungus and ant-bacterium mutualisms, mixing of genetically distinct strains can lead to antagonistic interactions (i.e., competitive conflict), which may prevent the ants from rearing multiple strains of either of the mutualistic symbionts within individual colonies. The success of different ant-cultivar-bacterium combinations could ultimately be governed by antagonistic interactions between the two mutualists, either as inhibition of the cultivar by Pseudonocardia or vice versa. Here we explore cultivar-Pseudonocardia antagonism by evaluating in vitro interactions between strains of the two mutualists, and find frequent antagonistic interactions both from cultivars towards Pseudonocardia and vice versa. To test whether such in vitro antagonistic interactions affect ant colonies in vivo, we performed sub-colony experiments using species of Acromyrmex leaf-cutting ants. We created novel ant-fungus-bacterium pairings in which there was antagonism from one, both, or neither of the ants' microbial mutualists, and evaluated the effect of directional antagonism on cultivar biomass and Pseudonocardia abundance on the cuticle of workers within sub-colonies. Despite the presence of frequent in vitro growth suppression between cultivars and Pseudonocardia, antagonism from Pseudonocardia towards the cultivar did not reduce sub-colony fungus garden biomass, nor did cultivar antagonism towards Pseudonocardia reduce bacteria abundance on the cuticle of sub-colony workers. Our findings suggest that inter-mutualist antagonism does not limit what combinations of cultivar and Pseudonocardia strains Acromyrmex fungus-growing ants can maintain within nests.
Conflict of interest statement
Figures






Similar articles
-
Antagonistic bacterial interactions help shape host-symbiont dynamics within the fungus-growing ant-microbe mutualism.PLoS One. 2007 Sep 26;2(9):e960. doi: 10.1371/journal.pone.0000960. PLoS One. 2007. PMID: 17896000 Free PMC article.
-
Symbiont-Mediated Protection of Acromyrmex Leaf-Cutter Ants from the Entomopathogenic Fungus Metarhizium anisopliae.mBio. 2021 Dec 21;12(6):e0188521. doi: 10.1128/mBio.01885-21. Epub 2021 Dec 21. mBio. 2021. PMID: 34933458 Free PMC article.
-
The role of symbiont genetic distance and potential adaptability in host preference towards Pseudonocardia symbionts in Acromyrmex leaf-cutting ants.J Insect Sci. 2011;11:120. doi: 10.1673/031.011.12001. J Insect Sci. 2011. PMID: 22225537 Free PMC article.
-
Chemical warfare between fungus-growing ants and their pathogens.Curr Opin Chem Biol. 2020 Dec;59:172-181. doi: 10.1016/j.cbpa.2020.08.001. Epub 2020 Sep 17. Curr Opin Chem Biol. 2020. PMID: 32949983 Free PMC article. Review.
-
Pseudonocardia Symbionts of Fungus-Growing Ants and the Evolution of Defensive Secondary Metabolism.Front Microbiol. 2020 Dec 22;11:621041. doi: 10.3389/fmicb.2020.621041. eCollection 2020. Front Microbiol. 2020. PMID: 33424822 Free PMC article. Review.
Cited by
-
The genome of the leaf-cutting ant Acromyrmex echinatior suggests key adaptations to advanced social life and fungus farming.Genome Res. 2011 Aug;21(8):1339-48. doi: 10.1101/gr.121392.111. Epub 2011 Jun 30. Genome Res. 2011. PMID: 21719571 Free PMC article.
-
Paenibacillus polymyxa Associated with the Stingless Bee Melipona scutellaris Produces Antimicrobial Compounds against Entomopathogens.J Chem Ecol. 2018 Dec;44(12):1158-1169. doi: 10.1007/s10886-018-1028-z. Epub 2018 Oct 23. J Chem Ecol. 2018. PMID: 30350228
-
Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani.PLoS Negl Trop Dis. 2019 Aug 5;13(8):e0007643. doi: 10.1371/journal.pntd.0007643. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31381572 Free PMC article.
-
The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi.Sci Rep. 2013 Nov 19;3:3250. doi: 10.1038/srep03250. Sci Rep. 2013. PMID: 24248063 Free PMC article.
-
Context-dependent symbioses and their potential roles in wildlife diseases.Proc Biol Sci. 2012 Apr 22;279(1733):1457-65. doi: 10.1098/rspb.2011.2276. Epub 2012 Jan 11. Proc Biol Sci. 2012. PMID: 22237907 Free PMC article. Review.
References
-
- Margulis L. Symbiosis in cell evolution. W.H. Freeman & Co., New York, NY. 1981
-
- Baldauf SL. The deep roots of eukaryotes. Science. 2003;300:1703–1706. - PubMed
-
- Moran NA. Symbiosis. Curr Biol. 2006;16:R866. - PubMed
-
- Douglas AE. Symbiotic Interactions. Oxford University Press, Oxford. 1994
-
- Herre EA, Knowlton N, Mueller UG, Rehner SA. The evolution of mutualisms: exploring the paths between conflicts and cooperation. Trends Ecol Evol. 1999;14:49–53. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

