Cardiac sodium channelopathies
- PMID: 20091048
- PMCID: PMC2883928
- DOI: 10.1007/s00424-009-0761-0
Cardiac sodium channelopathies
Abstract
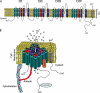
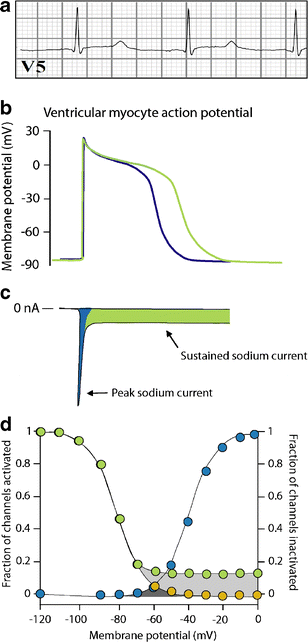
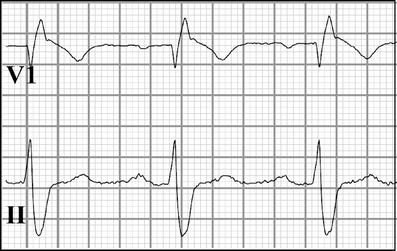
Cardiac sodium channel are protein complexes that are expressed in the sarcolemma of cardiomyocytes to carry a large inward depolarizing current (INa) during phase 0 of the cardiac action potential. The importance of INa for normal cardiac electrical activity is reflected by the high incidence of arrhythmias in cardiac sodium channelopathies, i.e., arrhythmogenic diseases in patients with mutations in SCN5A, the gene responsible for the pore-forming ion-conducting alpha-subunit, or in genes that encode the ancillary beta-subunits or regulatory proteins of the cardiac sodium channel. While clinical and genetic studies have laid the foundation for our understanding of cardiac sodium channelopathies by establishing links between arrhythmogenic diseases and mutations in genes that encode various subunits of the cardiac sodium channel, biophysical studies (particularly in heterologous expression systems and transgenic mouse models) have provided insights into the mechanisms by which INa dysfunction causes disease in such channelopathies. It is now recognized that mutations that increase INa delay cardiac repolarization, prolong action potential duration, and cause long QT syndrome, while mutations that reduce INa decrease cardiac excitability, reduce electrical conduction velocity, and induce Brugada syndrome, progressive cardiac conduction disease, sick sinus syndrome, or combinations thereof. Recently, mutation-induced INa dysfunction was also linked to dilated cardiomyopathy, atrial fibrillation, and sudden infant death syndrome. This review describes the structure and function of the cardiac sodium channel and its various subunits, summarizes major cardiac sodium channelopathies and the current knowledge concerning their genetic background and underlying molecular mechanisms, and discusses recent advances in the discovery of mutation-specific therapies in the management of these channelopathies.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

