Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death
- PMID: 20091232
- PMCID: PMC2845893
- DOI: 10.1007/s10495-009-0436-5
Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death
Abstract
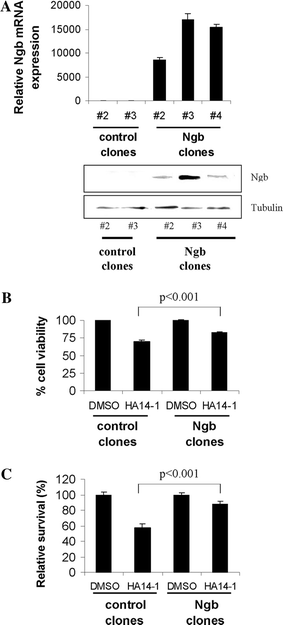
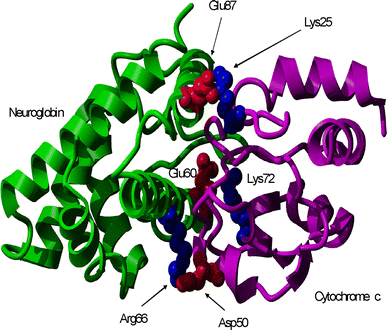
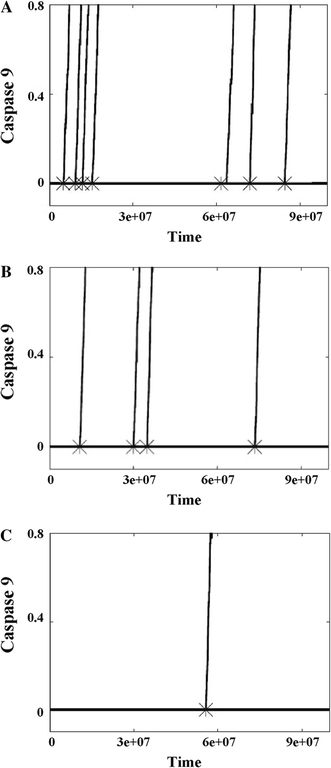
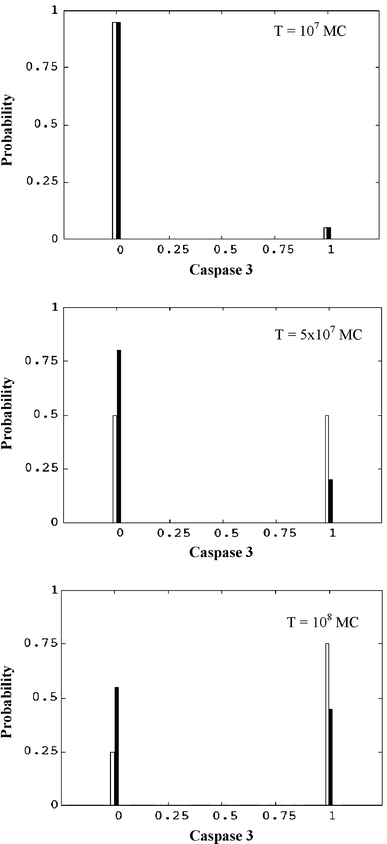
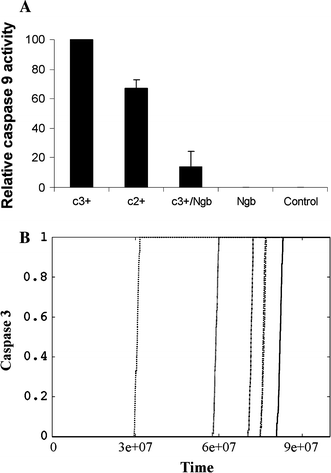
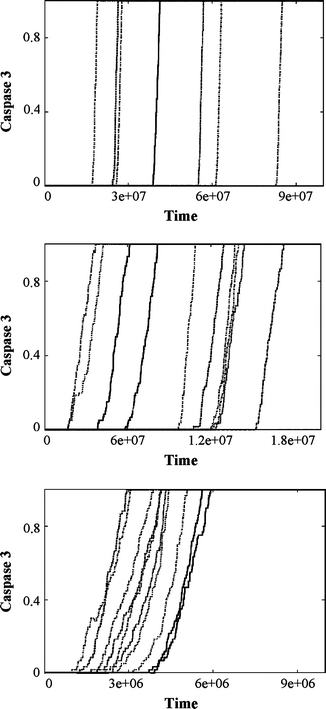
In the past few years, overwhelming evidence has accrued that a high level of expression of the protein neuroglobin protects neurons in vitro, in animal models, and in humans, against cell death associated with hypoxic and amyloid insult. However, until now, the exact mechanism of neuroglobin's protective action has not been determined. Using cell biology and biochemical approaches we demonstrate that neuroglobin inhibits the intrinsic pathway of apoptosis in vitro and intervenes in activation of pro-caspase 9 by interaction with cytochrome c. Using systems level information of the apoptotic signalling reactions we have developed a quantitative model of neuroglobin inhibition of apoptosis, which simulates neuroglobin blocking of apoptosome formation at a single cell level. Furthermore, this model allows us to explore the effect of neuroglobin in conditions not easily accessible to experimental study. We found that the protection of neurons by neuroglobin is very concentration sensitive. The impact of neuroglobin may arise from both its binding to cytochrome c and its subsequent redox reaction, although the binding alone is sufficient to block pro-caspase 9 activation. These data provides an explanation the action of neuroglobin in the protection of nerve cells from unwanted apoptosis.
Figures
Similar articles
-
Extended survival of SH-SY5Y cells following overexpression of Lys67Glu neuroglobin is associated with stabilization of ΔψM.Cytometry A. 2012 Jul;81(7):602-10. doi: 10.1002/cyto.a.22046. Epub 2012 Mar 29. Cytometry A. 2012. PMID: 22467552
-
Neuroglobin Attenuates Beta Amyloid-Induced Apoptosis Through Inhibiting Caspases Activity by Activating PI3K/Akt Signaling Pathway.J Mol Neurosci. 2016 Jan;58(1):28-38. doi: 10.1007/s12031-015-0645-z. Epub 2015 Sep 7. J Mol Neurosci. 2016. PMID: 26346601
-
An antiapoptotic neuroprotective role for neuroglobin.Int J Mol Sci. 2010 May 27;11(6):2306-21. doi: 10.3390/ijms11062306. Int J Mol Sci. 2010. PMID: 20640154 Free PMC article. Review.
-
Neuroglobin upregulation induced by 17β-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells.Cell Death Dis. 2013 Feb 21;4(2):e508. doi: 10.1038/cddis.2013.30. Cell Death Dis. 2013. PMID: 23429294 Free PMC article.
-
Neuroglobin, a Factor Playing for Nerve Cell Survival.Int J Mol Sci. 2016 Oct 31;17(11):1817. doi: 10.3390/ijms17111817. Int J Mol Sci. 2016. PMID: 27809238 Free PMC article. Review.
Cited by
-
Neuroglobin mitigates mitochondrial impairments induced by acute inhalation of combustion smoke in the mouse brain.Inhal Toxicol. 2014 May;26(6):361-9. doi: 10.3109/08958378.2014.902147. Inhal Toxicol. 2014. PMID: 24730682 Free PMC article.
-
Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury.Sci Rep. 2014 Nov 18;4:7091. doi: 10.1038/srep07091. Sci Rep. 2014. PMID: 25404538 Free PMC article.
-
Five-coordinate H64Q neuroglobin as a ligand-trap antidote for carbon monoxide poisoning.Sci Transl Med. 2016 Dec 7;8(368):368ra173. doi: 10.1126/scitranslmed.aah6571. Sci Transl Med. 2016. PMID: 27928027 Free PMC article.
-
Neuroglobin protection in retinal ischemia.Invest Ophthalmol Vis Sci. 2012 Feb 13;53(2):704-11. doi: 10.1167/iovs.11-7408. Print 2012 Feb. Invest Ophthalmol Vis Sci. 2012. PMID: 22167093 Free PMC article.
-
Hypoxic regulation of cytoglobin and neuroglobin expression in human normal and tumor tissues.Cancer Cell Int. 2010 Sep 9;10:33. doi: 10.1186/1475-2867-10-33. Cancer Cell Int. 2010. PMID: 20828399 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

