Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants
- PMID: 20091513
- PMCID: PMC7059181
- DOI: 10.1002/14651858.CD001079.pub2
Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants
Abstract
Background: Respiratory distress syndrome (RDS) is caused by a deficiency or dysfunction of pulmonary surfactant. A variety of surfactant products including protein free synthetic surfactant have been developed and tested in the prevention and treatment of RDS.
Objectives: To assess the effect of prophylactic administration of protein free synthetic surfactant (SS) on mortality, chronic lung disease and other morbidities associated with prematurity in preterm newborns at risk for developing RDS. Subgroup analysis were planned according to the degree of prematurity, surfactant product and dosage schedule.
Search strategy: Searches were made of the The Cochrane Library, MEDLINE, OVID, EMBASE, CINAHL from 1966 to 2009. In addition, previous reviews including cross references and abstracts from the Society for Pediatric Research were searched. No language restrictions were applied.
Selection criteria: Randomized and quasi-randomized controlled trials that compared the effect of protein free SS administered to high risk preterm newborns at or shortly after birth in order to prevent RDS, mortality and complications of prematurity.
Data collection and analysis: Data regarding clinical outcomes was excerpted from the clinical trials by the reviewers. Data were analyzed according to the standards of the Cochrane Neonatal Review Group.
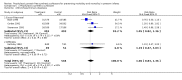
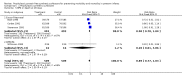
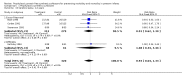
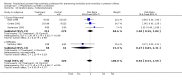
Main results: Studies of prophylactic administration of protein free SS note a variable improvement in the respiratory status and a decrease in respiratory distress syndrome in infants who receive prophylactic protein free SS. The meta-analysis supports a decrease in the risk of pneumothorax (typical relative risk 0.67, 95% CI 0.50, 0.90), pulmonary interstitial emphysema (typical relative risk 0.68, 95% CI 0.50, 0.93), and neonatal mortality (typical relative risk 0.70, 95% CI 0.58, 0.85). No differences were seen in the risk of intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity and cerebral palsy. The meta-analysis supports an increase in the risk of patent ductus arteriosus associated with prophylactic SS administration (typical relative risk 1.11, 95% CI 1.00, 1.22), and an increase in the risk of pulmonary hemorrhage (typical relative risk 3.28, 95% CI 1.50, 7.16).
Authors' conclusions: Prophylactic intratracheal administration of protein free synthetic surfactant to infants at risk of developing respiratory distress syndrome has been demonstrated to improve clinical outcome. Infants who receive prophylactic protein free SS have a decreased risk of pneumothorax, a decreased risk of pulmonary interstitial emphysema, and a decreased risk of neonatal mortality. Infants who receive prophylactic protein free SS have an increased risk of developing patent ductus arteriosus and pulmonary hemorrhage.
Conflict of interest statement
Dr. R. Soll has acted as a consultant and invited speaker for several of the pharmaceutical companies which manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs‐Welcome).
Figures













Update of
-
Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2000;(2):CD001079. doi: 10.1002/14651858.CD001079. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD001079. doi: 10.1002/14651858.CD001079.pub2. PMID: 10796410 Updated.
References
References to studies included in this review
Bose 1990 {published data only}
-
- Bose C, Corbet A, Bose G, Garcia‐Prats J, Lombardy L, Wold D, Donlon D, Long W. Improved outcome at 28 days of age for very low birthweight infants treated with a single dose of a synthetic surfactant. Journal of Pediatrics 1990;117:947‐53. - PubMed
-
- Corbet A, Long W, Schumacher R, et al. Double‐blind developmental evaluation at one year corrected age of 597 premature infants with birthweights from 500 to 1350 grams enrolled in three placebo controlled trials of prophylactic synthetic surfactant. Journal of Pediatrics 1995;126:S5‐12. - PubMed
-
- Kraybill EN, Bose C, Corbet A, Garcia‐Prats J, Asbill D, Edwards K, Long W. Double‐blind evaluation of developmental and health status to age 2 years of infants weighing 700 to 1350 grams treated prophylactically at birth with a single dose of synthetic surfactant or air placebo. Journal of Pediatrics 1995;126:S33‐42. - PubMed
Corbet 1991 {published data only}
-
- Corbet A, Bucciarelli R, Goldman S, Mammel M, Wold D, Long W, US Exosurf Pediatric Study Group 1. Decreased mortality rate among small premature infants treated at birth with a single dose of synthetic surfactant: a multicenter controlled trial. Journal of Pediatrics 1991;118:277‐84. - PubMed
-
- Corbet A, Long W, Schumacher R, et al. Double‐blind developmental evaluation at one year corrected age of 597 premature infants with birthweights from 500 to 1350 grams enrolled in three placebo controlled trials of prophylactic synthetic surfactant. Journal of Pediatrics 1995;126:S5‐12. - PubMed
-
- Sell M, Cotton R, Hirata T, Guthrie R, Leblanc M, Mammel M, Long W. One year follow‐up of 273 infants with birthweights of 700 to 1100 grams after prophylactic treatment of respiratory distress syndrome with synthetic surfactant or air placebo. Journal of Pediatrics 1995;126:S20‐25. - PubMed
Halliday 1984 {published data only}
-
- Halliday HL, McClure G, Reid MM. Growth and development two years after artificial surfactant replacement at birth. Early Human Development 1986;13:323‐7. - PubMed
-
- Halliday HL, Reid MM, Meban C, McClure G, Lappin TRJ, Thomas PS. Controlled trial of artificial surfactant to prevent respiratory distress syndrome. Lancet 1984;1:476‐8. - PubMed
Phibbs 1991 {published data only}
-
- Phibbs RH, Ballard RA, Clements JA, Heilbron DC, Phibbs CS, Schlueter MA, Sniderman SH, Tooley WH, Wakeley A. Initial clinical trial of Exosurf, a protein‐free synthetic surfactant, for the prophylaxis and early treatment of hyaline membrane disease. Pediatrics 1991;88:1‐9. - PubMed
Stevenson 1992 {published data only}
-
- Stevenson D, Walther F, Long W, Sell M, Pauly T, Gong A, Easa D, Pramanik A, LeBlanc M, Anday E, Dhanireddy R, Burchfield D, Corbet A, American Exosurf Neonatal Study Group 1. Controlled trial of a single dose of synthetic surfactant at birth in premature infants weighing 500 to 699 grams. Journal of Pediatrics 1992;120:S3‐S12. - PubMed
-
- Walther FJ, Mullett M, Schumacher R, Sundell H, Easa D, Long W. One year follow‐up of 66 premature infants weighing 500 to 699 grams treated with a single dose of synthetic surfactant or air placebo at birth: results of a double‐blind trial. Journal of Pediatrics 1995;126:S13‐9. - PubMed
Ten Centre 1987 {published data only}
Wilkinson 1985 {published data only}
-
- Wilkinson AR, Jenkins PA, Jeffrey JA. Two controlled trials of dry artificial surfactant: early effects and later outcome in babies with surfactant deficiency. Lancet 1985;2:287‐91. - PubMed
References to studies excluded from this review
Chu 1967 {published data only}
-
- Chu J, Clements JA, Cotten EK, Klaus MH, Sweet AY, Tooley WH. Neonatal Pulmonary Ischemia. Pediatrics 1967;40 Suppl:709‐82. - PubMed
Milner 1984 {published data only}
Morley 1981 {published data only}
-
- Morley CJ, Bangham AD, Miller N, Davis JA. Dry artificial surfactant and its effect on very premature babies. Lancet 1981;i:64‐8. - PubMed
Morley 1988 {published data only}
-
- Morley CJ, Greenough A, Miller NG, Bangham AD, Pool J, Wood S, South M, Davis JA, Vyas H. Randomized trial of artificial surfactant (ALEC) gien at birth to babies from 23 to 34 weeks gestation. Early Human Development 1988;17:41‐54. - PubMed
Morley 1991 {published data only}
Additional references
Enhorning 1972
-
- Enhorning G, Robertson B. Lung expansion in the premature rabbit fetus after tracheal deposition of surfactant. Pediatrics 1972;50:58‐66. - PubMed
Jobe 1993
-
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328:861‐8. - PubMed
Pfister 2007
Raju 1993
-
- Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous surfactant. Journal of Pediatrics 1993;123:603‐10. - PubMed
Seger 2009
Soll 1998a
Soll 1998b
Soll 2001a
Soll 2001b
Stevens 2007
-
- Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] - DOI - PMC - PubMed
Tooley 1987
-
- Tooley WH, Clements JA, Maramatsu K, Brown CL, Schlueter MA. Lung function in prematurely delivered rabbits treated with a synthetic surfactant. American Review of Respiratory Disease 1987;136:651‐6. - PubMed
References to other published versions of this review
Soll 1992
-
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:325‐58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

