Dietary interventions for phenylketonuria
- PMID: 20091517
- PMCID: PMC7092572
- DOI: 10.1002/14651858.CD001304.pub2
Dietary interventions for phenylketonuria
Update in
-
Dietary interventions for phenylketonuria.Cochrane Database Syst Rev. 2020 Jul 16;7(7):CD001304. doi: 10.1002/14651858.CD001304.pub3. Cochrane Database Syst Rev. 2020. PMID: 32672365 Free PMC article.
Abstract
Background: Phenylketonuria is an inherited disease treated with dietary restriction of the amino acid phenylalanine. The diet is initiated in the neonatal period to prevent mental handicap; however, it is restrictive and can be difficult to follow. Whether the diet can be relaxed or discontinued during adolescence or should be continued for life remains a controversial issue, which we aim to address in this review.
Objectives: To assess the effects of a low-phenylalanine diet commenced early in life for people with phenylketonuria. To assess the possible effects of relaxation or termination of the diet on intelligence, neuropsychological outcomes and mortality, growth, nutritional status, eating behaviour and quality of life.
Search strategy: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings.Most recent search of the Inborn Errors of Metabolism Trials Register: 05 March 2009.
Selection criteria: All randomised or quasi-randomised controlled trials comparing a low-phenylalanine diet to relaxation or termination of dietary restrictions in people with phenylketonuria.
Data collection and analysis: Two authors independently assessed study eligibility and methodological quality, and subsequently extracted the data.
Main results: We included four studies in this review (251 participants), and found few significant differences between treatment and comparison groups for the outcomes of interest. Blood phenylalanine levels were significantly lower in participants with phenylketonuria following a low-phenylalanine diet compared to those on a less restricted diet, mean difference (MD) at three months -698.67 (95% confidence interval (CI) -869.44 to -527.89). Intelligence quotient was significantly higher in participants who continued the diet than in those who stopped the diet, MD after 12 months 5.00 (95% CI 0.40 to 9.60). However, these results came from a single study.
Authors' conclusions: The results of non-randomised studies have concluded that a low-phenylalanine diet is effective in reducing blood phenylalanine levels and improving intelligence quotient and neuropsychological outcomes. We were unable to find any randomised controlled studies that have assessed the effect of a low-phenylalanine diet versus no diet from diagnosis. In view of evidence from non-randomised studies, such a study would be unethical and it is recommended that low-phenylalanine diet should be commenced at the time of diagnosis. There is uncertainty about the precise level of phenylalanine restriction and when, if ever, the diet should be relaxed. This should be addressed by randomised controlled studies.
Conflict of interest statement
Vanessa Poustie and Patricia Rutherford have received travel expenses to attend conferences from the manufacturers of dietary products used in the treatment of PKU.
Figures




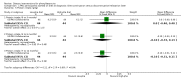





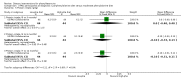
Update of
-
Dietary interventions for phenylketonuria.Cochrane Database Syst Rev. 2000;(2):CD001304. doi: 10.1002/14651858.CD001304. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD001304. doi: 10.1002/14651858.CD001304.pub2. PMID: 10796766 Updated.
Similar articles
-
Dietary interventions for phenylketonuria.Cochrane Database Syst Rev. 2000;(2):CD001304. doi: 10.1002/14651858.CD001304. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD001304. doi: 10.1002/14651858.CD001304.pub2. PMID: 10796766 Updated.
-
Tyrosine supplementation for phenylketonuria.Cochrane Database Syst Rev. 2010 Aug 4;(8):CD001507. doi: 10.1002/14651858.CD001507.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 05;(6):CD001507. doi: 10.1002/14651858.CD001507.pub3. PMID: 20687067 Updated.
-
Tyrosine supplementation for phenylketonuria.Cochrane Database Syst Rev. 2013 Jun 5;2013(6):CD001507. doi: 10.1002/14651858.CD001507.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2021 Jan 4;1:CD001507. doi: 10.1002/14651858.CD001507.pub4. PMID: 23737086 Free PMC article. Updated.
-
Tyrosine supplementation for phenylketonuria.Cochrane Database Syst Rev. 2000;(2):CD001507. doi: 10.1002/14651858.CD001507. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2010 Aug 04;(8):CD001507. doi: 10.1002/14651858.CD001507.pub2. PMID: 10796799 Updated.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
The personal burden for caregivers of children with phenylketonuria: A cross-sectional study investigating time burden and costs in the UK.Mol Genet Metab Rep. 2016 Aug 28;9:1-5. doi: 10.1016/j.ymgmr.2016.08.008. eCollection 2016 Dec. Mol Genet Metab Rep. 2016. PMID: 27622144 Free PMC article.
-
No solution yet for combining two independent studies in the presence of heterogeneity.Stat Med. 2015 Jul 20;34(16):2476-80. doi: 10.1002/sim.6473. Stat Med. 2015. PMID: 26040434 Free PMC article. No abstract available.
-
Health Related Quality of Life of Caregivers of Children and Adolescents With Phenylketonuria: A Systematic Review.Glob Pediatr Health. 2021 Dec 17;8:2333794X211065333. doi: 10.1177/2333794X211065333. eCollection 2021. Glob Pediatr Health. 2021. PMID: 34950751 Free PMC article. Review.
-
Phenylketonuria.Nat Rev Dis Primers. 2021 May 20;7(1):36. doi: 10.1038/s41572-021-00267-0. Nat Rev Dis Primers. 2021. PMID: 34017006 Free PMC article. Review.
-
Quality of life and adherence to treatment in early-treated Brazilian phenylketonuria pediatric patients.Braz J Med Biol Res. 2017 Dec 11;51(2):e6709. doi: 10.1590/1414-431X20176709. Braz J Med Biol Res. 2017. PMID: 29267500 Free PMC article.
References
References to studies included in this review
Clarke 1987 {published data only}
-
- Clarke JTR, Gates RD, Hogan SE, Barrett M, MacDonald GW. Neuropsychological studies on adolescents with phenylketonuria returned to phenylalanine‐restricted diets. American Journal of Mental Retardation 1987;92(3):255‐62. - PubMed
Griffiths 1998 {published data only}
-
- Griffiths P, Smith C, Harvie A. Transitory hyperphenylalaninaemia in children with continuously treated phenylketonuria. American Journal of Mental Retardation 1997;102(1):27‐36. - PubMed
-
- Griffiths P, Ward N, Harvie A, Cockburn F. Neuropsychological outcome of experimental manipulation of phenylalanine intake in treated phenylketonuria. Journal of Inherited Metabolic Disease 1998;21(1):29‐38. - PubMed
Holtzman 1975 {published data only}
-
- Holtzman NA, Welcher DW, Mellits ED. Termination of restricted diet in children with phenylketonuria: a randomised controlled study. New England Journal of Medicine 1975;293(22):1121‐4. - PubMed
US/PKU Collaborative {published data only}
-
- Acosta PB, Trahams C, Wellman NS, Williamson M. Phenylalanine intakes of 1‐ to 6‐year old children with phenylketonuria undergoing therapy. American Journal of Clinical Nutrition 1983;38(5):694‐700. - PubMed
-
- Acosta PB, Wentz E, Williamson M. Nutrient intake of treated infants with phenylketonuria. American Journal of Clinical Nutrition 1977;30(2):198‐208. - PubMed
-
- Azen C, Koch R, Friedman E, Wenz E, Fishler K. Summary of findings from the United States Collaborative Study of children treated for phenylketonuria. European Journal of Pediatrics 1996;155(Suppl 1):S29‐S32. - PubMed
-
- Azen CG, Koch R, Friedman EG, Berlow S, Coldwell J, Krause W, et al. Intellectual development in 12‐year‐old children treated for phenylketonuria. American Journal of Diseases of Children 1991;145(1):35‐9. - PubMed
-
- Dobson JC, Williamson ML, Azen C, Koch R. Intellectual assessment of 111 four‐year‐old children with phenylketonuria. Pediatrics 1977;60(6):822‐7. - PubMed
References to studies excluded from this review
Bentovim 1968 {published data only}
Cleary 2006 {published data only}
-
- Cleary MA, Feillet F, White FJ, Vidailhet M, Macdonald A, Grimsley A, et al. Randomised controlled trial of essential fatty acid supplementation in phenylketonuria. European Journal of Clinical Nutrition 2006;60(7):915‐20. [MEDLINE: ] - PubMed
Epstein 1989 {published data only}
-
- Epstein CM, Trotter JF, Averbook A, Freeman S, Kutner MH, Elsas LJ. EEG mean frequencies are sensitive indices of phenylalanine effects on normal brain. Electroencephalography and Clinical Neurophysiology 1989;72(2):133‐9. - PubMed
Frankenburg 1973 {published data only}
-
- Frankenburg WK, Goldstein AD. Behavioural consequences of increased phenylalanine intake by phenylketonuric children: a pilot study describing a methodology. American Journal of Mental Deficiency 1973;77(5):524‐32. - PubMed
Kakanoglu 2005 {published data only}
-
- Kalkanoglu HS, Ahring KK, Sertkaya D, Moller LB, Romstad A, Mikkelsen I, et al. Behavioural effects of phenylalanine‐free amino acid tablet supplementation in intellectually disabled adults with untreated phenylketonuria. Act Paediatrica 2005;94(9):1218‐22. - PubMed
Koff 1979 {published data only}
-
- Koff E, Kammerer B, Boyle P, Pueschel SM. Intelligence and phenylketonuria: effects of diet termination. Journal of Pediatrics 1979;94(4):534‐7. - PubMed
Koletzko 2007 {published data only}
-
- Koletzko B, Sauerwald T, Demmelmair H, Herzog M, Schenck U, Bohles H, et al. Dietary long‐chain polyunsaturated fatty acid supplementation in infants with phenylketonuria: a randomized controlled trial. Journal of Inherited Metabolic Disease 2007;30(3):326‐32. - PubMed
Krause 1985 {published data only}
-
- Krause W, Halminski M, McDonald L, Dembure P, Salvo R, Freides D, et al. Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with treated phenylketonuria. A model for the study of phenylalanine and brain function in man.. Journal of Clinical Investigation 1985;75(1):40‐8. - PMC - PubMed
Levy 2007 {published data only}
-
- Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R‐BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo‐controlled study. Lancet 2007;370(9586):504‐10. - PubMed
Lou 1987 {published data only}
-
- Lou HC, Lykkelund C, Gerdes AM, Udesen H, Bruhn P. Increased vigilance and dopamine synthesis by large doses of tyrosine or phenylalanine restriction in phenylketonuria. Acta Paediatrica Scandinavica 1987;76(4):560‐5. [MEDLINE: ] - PubMed
Pietz 1995 {published data only}
-
- Pietz J, Landwehr R, Kutscha A, Schmidt H, Sonneville L, Trefz FK. Effect of high‐dose tyrosine supplementation on brain function in adults with phenylketonuria. Journal of Pediatrics 1995;127(6):936‐43. [MEDLINE: ] - PubMed
Realmuto 1986 {published data only}
-
- Realmuto GM, Garfinkel BD, Tuchman M, Tsai MY, Chang PN, Fisch RO, et al. Psychiatric diagnosis and behavioural characteristics of phenylketonuric children. Journal of Nervous and Mental Disease 1986;174(9):536‐40. - PubMed
Rey 1996 {published data only}
-
- Rey F, Abadie V, Plainguet F, Rey J. Long‐term follow up of patients with classical phenylketonuria after diet relaxation at 5 years of age. European Journal of Pediatrics 1996;155(Suppl 1):S39‐S44. - PubMed
Robertson 2004 {published data only}
-
- Robertson L, Lee P, Murphy G, Amos A. A trial to assess the effectiveness of a low phenylalanine diet in adults with previously untreated phenylketonuria (PKU) [abstract]. SHS Inborn Error Review Series (Dietary Management of Inborn Errors of Metabolic Disease) 2004;14:10.
-
- Robertson L, Lee P, Murphy G, Amos A. A trial to assess the effectiveness of a low phenylalanine diet in adults with previously untreated phenylketonuria (PKU) [abstract]. SHS Inborn Error Review Series (Dietary Management of Inborn Errors of Metabolic Disease) 2004;14:18.
-
- Robertson L, Murphy G, Amos A, Lee P, Dent C. A randomised control trial of diet in adults with previously untreated phenylketonuria (PKU) [abstract]. Dietary Management of Inherited Metabolic Disease (DMIMD); 2008 April 17‐18th; London, UK. 2008:36. [MEDLINE: ]
Schindeler 2007 {published data only}
-
- Schindeler S, Ghosh‐Jerath S, Thompson S, Rocca A, Joy P, Kemp A, et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Molecular Genetics and Metabolism 2007;91(1):48‐54. - PubMed
Schmidt 1994 {published data only}
-
- Schmidt E, Rupp A, Burgard P, Pietz J, Weglage J, Sonneville L. Sustained attention in adult phenylketonuria: the influence of the concurrent phenylalanine blood level. Journal of Clinical Experimental Neuropsychology 1994;16(5):681‐8. - PubMed
Schmidt 1996 {published data only}
-
- Schmidt E, Burgard P, Rupp A. Effects of concurrent phenylalanine levels on sustained attention and calculation speed in patients treated early for phenylketonuria. European Journal of Pediatrics 1996;155(Suppl 1):S82‐S86. - PubMed
Additional references
Bickel 1953
-
- Bickel H. Influence of phenylalanine intake on phenylketonuria. Lancet 1953;2:812‐3. - PubMed
Dixon 1994
-
- Dixon M. Disorders of amino acid metabolism, organic acidaemias and uria cycle defects. In: Shaw V, Lawson M editor(s). Clinical Paediatric Dietetics. 1st Edition. Oxford: Blackwell Science, 1994:177‐209.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Fisch 1997
-
- Fisch RO, Matalon R, Weisberg S, Michals K. Phenylketonuria: Current dietary treatment practices in the United States and Canada. Journal of the American College of Nutrition 1997;16(2):147‐51. - PubMed
Michals 1988
-
- Michals K, Azen C, Acosta P, Koch R, Matalon R. Blood phenylalanine levels and intelligence of 10 year old children with PKU in the National Collaborative Study. Journal of the American Dietetic Association 1988;88(10):1226‐9. - PubMed
MRC1 1993
MRC2 1993
Paine 1957
-
- Paine RS. The variability and manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria). Pediatrics 1957;20:290‐302. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. - PubMed
Scriver 1995
-
- Scriver CR, Kaufman S, Woo SLC. The hyperphenylalaninaemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D editor(s). The Metabolic and Molecular Bases of Inherited Disease. 7th Edition. New York: McGraw‐Hil, 1995:1015‐76.
Smith 1994
-
- Smith I. Treatment of phenylketonuria hydroxylase deficiency. Acta Paediatrica Supplement 1994;407:60‐5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

