Antithyroid drug regimen for treating Graves' hyperthyroidism
- PMID: 20091544
- PMCID: PMC6599817
- DOI: 10.1002/14651858.CD003420.pub4
Antithyroid drug regimen for treating Graves' hyperthyroidism
Abstract
Background: Antithyroid drugs are widely used in the therapy of hyperthyroidism. There are wide variations in the dose, regimen or duration of treatment used by health professionals.
Objectives: To assess the effects of dose, regimen and duration of antithyroid drug therapy for Graves' hyperthyroidism.
Search strategy: We searched seven databases and reference lists.
Selection criteria: Randomised and quasi-randomised trials of antithyroid medication for Graves' hyperthyroidism.
Data collection and analysis: Two authors independently extracted data and assessed risk of bias. Pooling of data for primary outcomes, and select exploratory analyses were undertaken.
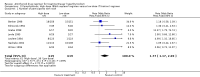
Main results: Twenty-six randomised trials involving 3388 participants were included. Overall the quality of trials, as reported, was poor. None of the studies investigated incidence of hypothyroidism, changes in weight, health-related quality of life, ophthalmopathy progression or economic outcomes. Four trials examined the effect of duration of therapy on relapse rates, and when using the titration regimen 12 months was superior to six months, but there was no benefit in extending treatment beyond 18 months. Twelve trials examined the effect of block-replace versus titration block-regimens. The relapse rates were similar in both groups at 51% in the block-replace group and 54% in the titration block-group (OR 0.86, 95% confidence interval (CI) 0.68 to1.08) though adverse effects (rashes (10% versus 6%) and withdrawing due to side effects (16% versus 9%)) were significantly higher in the block-replace group. Three studies considered the addition of thyroxine with continued low dose antithyroid therapy after initial therapy with antithyroid drugs. There was significant heterogeneity between the studies and the difference between the two groups was not significant (OR 0.58, 95% CI 0.05 to 6.21). Four studies considered the addition of thyroxine alone after initial therapy with antithyroid drugs. There was no significant difference in the relapse rates between the groups after 12 months follow-up (OR 1.15, 95% CI 0.79 to 1.67). Two studies considered the addition of immunosuppressive agents. The results which were in favour of the interventions would need to be validated in other populations.
Authors' conclusions: The evidence suggests that the optimal duration of antithyroid drug therapy for the titration regimen is 12 to 18 months. The titration (low dose) regimen had fewer adverse effects than the block-replace (high dose) regimen and was no less effective. Continued thyroxine treatment following initial antithyroid therapy does not appear to provide any benefit in terms of recurrence of hyperthyroidism. Immunosuppressive therapies need further evaluation.
Conflict of interest statement
None known.
Figures
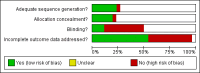






















Update of
-
Antithyroid drug regimen for treating Graves' hyperthyroidism.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003420. doi: 10.1002/14651858.CD003420.pub3. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003420. doi: 10.1002/14651858.CD003420.pub4. PMID: 15846664 Updated.
Similar articles
-
Antithyroid drug regimen for treating Graves' hyperthyroidism.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003420. doi: 10.1002/14651858.CD003420.pub3. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003420. doi: 10.1002/14651858.CD003420.pub4. PMID: 15846664 Updated.
-
Antithyroid drug regimen for treating Graves' hyperthyroidism.Cochrane Database Syst Rev. 2004;(2):CD003420. doi: 10.1002/14651858.CD003420.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003420. doi: 10.1002/14651858.CD003420.pub3. PMID: 15106202 Updated.
-
Antithyroid drug regimen for treating Graves' hyperthyroidism.Cochrane Database Syst Rev. 2003;(4):CD003420. doi: 10.1002/14651858.CD003420. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2004;(2):CD003420. doi: 10.1002/14651858.CD003420.pub2. PMID: 14583975 Updated.
-
Radioiodine therapy versus antithyroid medications for Graves' disease.Cochrane Database Syst Rev. 2016 Feb 18;2(2):CD010094. doi: 10.1002/14651858.CD010094.pub2. Cochrane Database Syst Rev. 2016. PMID: 26891370 Free PMC article.
-
A systematic review of drug therapy for Graves' hyperthyroidism.Eur J Endocrinol. 2005 Oct;153(4):489-98. doi: 10.1530/eje.1.01993. Eur J Endocrinol. 2005. PMID: 16189168
Cited by
-
Benefits of Long-Term Continuation of Low-Dose Methimazole Therapy in the Prevention of Recurrent Hyperthyroidism in Graves' Hyperthyroid Patients: A Randomized Prospective Controlled Study.Int J Endocrinol. 2022 Oct 11;2022:1705740. doi: 10.1155/2022/1705740. eCollection 2022. Int J Endocrinol. 2022. PMID: 36267362 Free PMC article.
-
The effect of COVID-19 process on patients with endocrinological disease in a pandemic hospital: What happened to the others?Arch Endocrinol Metab. 2023 Jan 18;67(1):45-54. doi: 10.20945/2359-3997000000525. Epub 2022 Oct 11. Arch Endocrinol Metab. 2023. PMID: 36219200 Free PMC article.
-
Toward Ultimate Care for Graves' Hyperthyroidism.Int J Endocrinol Metab. 2019 Nov 2;18(1):e98255. doi: 10.5812/ijem.98255. eCollection 2020 Jan. Int J Endocrinol Metab. 2019. PMID: 32308699 Free PMC article. No abstract available.
-
Serum TSH level as predictor of Graves' disease recurrence following antithyroid drug withdrawal: A systematic review.PLoS One. 2021 Jan 29;16(1):e0245978. doi: 10.1371/journal.pone.0245978. eCollection 2021. PLoS One. 2021. PMID: 33513181 Free PMC article.
-
A Study on Nonthermal Irreversible Electroporation of the Thyroid.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819876307. doi: 10.1177/1533033819876307. Technol Cancer Res Treat. 2019. PMID: 31564220 Free PMC article.
References
References to studies included in this review
Allannic 1990 {published data only}
-
- Allannic H, Fauchet R, Orgiazzi J, Madec AM, Genetet B, Lorcy Y, et al. Antithyroid drugs and Graves' disease: a prospective randomized evaluation of the efficacy of treatment duration. Journal of Clinical Endocrinology and Metabolism 1990;70:675‐9. - PubMed
-
- Allannic H, Lorcy Y, Leguerrier AM, Delambre C, Stetieh H, Madec AM, et al. Synthetic antithyroid drugs and Basedow's disease or the choice of a therapeutic strategy [Antithyrodiens de synthese et maladie de Basedow ou le choix d'une strategie therapeutique (French)]. Presse Medicale 1991;20(14):649‐51. - PubMed
Benker 1998 {published data only}
-
- Benker G, Reinwein D, Kahaly G, Tegler L, Alexander WD, Fabbinder J, et al. Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long‐term prospective study. The European Multicentre Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs. Clinical Endocrinology 1998;49(4):451‐7. [CN‐00304493] - PubMed
-
- Reinwein D, Benker G, Lazarus JH, Alexander WD and The European Multicentre Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs. A prospective randomized trial of antithyroid drug dose in Graves' disease therap [A prospective randomized trial of antithyroid drug dose in Graves' disease therapy]. Journal of Clinical Endocrinology and Metabolism 1993;76(6):1516‐21. - PubMed
Chen 2008 {published data only}
-
- Chen L, Wang H‐Q, Gao Y‐Y, Liang J, Wang M, Bai J, et al. Comparison of methimazole/hydrocortisone ointment with oral methimazole in patients with graves disease: A prospective, randomized, open‐label, parallel‐group, 18‐month study. Current Therapeutic Research ‐ Clinical and Experimental August 2008;69(4):305‐17. - PMC - PubMed
Cho 1992 {published data only}
-
- Cho BY, Shong MH, Yi KH, Lee HK, Koh CS, Min HK. Evaluation of serum basal thyrotrophin levels and thyrotrophin receptor antibody activities as prognostic markers for discontinuation of antithyroid drug treatment in patients with Graves' disease [Evaluation of serum basal thyrotrophin levels and thyrotrophin receptor antibody activities as prognostic markers for discontinuation of antithyroid drug treatment in patients with Graves' disease]. Clinical Endocrinology 1992;36(6):585‐90. - PubMed
Edmonds 1994 {published data only}
-
- Edmonds CJ. Personal Communication 01 September 2006.
-
- Edmonds CJ, Tellez M. Treatment of Graves' disease by carbimazole: high dose with thyroxine compared to titration dose. European Journal of Endocrinology 1994;131(2):120‐4. [MEDLINE: ; CN‐00104047] - PubMed
Garcia‐Mayor 1992 {published data only}
-
- Garcia‐Mayor RV, Paramo C, Luna Cano R, Perez Mendez LF, Galofre JC, Andrade A. Antithyroid drug and Graves' hyperthyroidism. Significance of treatment duration and TRAb determination on lasting remission [Antithyroid drug and Graves' hyperthyroidism. Significance of treatment duration and TRAb determination on lasting remission]. Journal of Endocrinology and Investigation 1992;15:815‐20. - PubMed
Glinoer 2001 {published data only}
-
- Glinoer D. Clinical epidemiology of Basedow's disease in Belgium [Epidemologie clinique de la maladie de Basedow en Belgique (French)]. Revue Medicale de Bruxelles 2000;21(4):A296‐9. - PubMed
-
- Glinoer D, Nayer P, Bex M, Belgian CS. Effects of l‐thyroxine administration, TSH‐receptor antibodies and smoking on the risk of recurrence in Graves' hyperthyroidism treated with antithyroid drugs: a double‐blind prospective randomized study [Effects of l‐thyroxine administration, TSH‐receptor antibodies and smoking on the risk of recurrence in Graves' hyperthyroidism treated with antithyroid drugs: a double‐blind prospective randomized study]. European Journal of Endocrinology 2001;144(5):475‐83. - PubMed
Goni Iriarte 1995 {published data only}
-
- Goni Iriarte MJ, Forga Llenas L, Iriarte Beroiz A, Apinaniz EA, Rodriguez Erdozain R, Menendez Torre E. Recurrence of Graves‐Basedow disease: the influence of treatment schedule [Recidivas en la enfermedad de Graves‐Basedow: influencia de la pauta de tratamiento (Spanish)]. Medicina Clinica 1995;104(1):11‐4. - PubMed
Grebe 1998 {published data only}
-
- Grebe SK, Feek CM, Ford HC, Fagerstrom JN, Cordwell DP, Delahunt JW, et al. A randomized trial of short‐term treatment of Graves' disease with high‐ dose carbimazole plus thyroxine versus low‐dose carbimazole [A randomized trial of short‐term treatment of Graves' disease with high‐ dose carbimazole plus thyroxine versus low‐dose carbimazole]. Clinical Endocrinology 1998;48(5):585‐92. - PubMed
Hashizume 1991 {published data only}
-
- Hashizume K, Ichikawa K, Sakurai A, Suzuki S, Takeda T, Kobayashi M, et al. Administration of thyroxine in treated Graves' disease. Effects on the level of antibodies to thyroid‐stimulating hormone receptors and on the risk of recurrence of hyperthyroidism [Administration of thyroxine in treated Graves' disease. Effects on the level of antibodies to thyroid‐stimulating hormone receptors and on the risk of recurrence of hyperthyroidism]. New England Journal of Medicine 1991;324(14):947‐53. - PubMed
Hoermann 2002 {published data only}
-
- Hoermann R, Quadbeck B, Roggenbuck U, Szabolcs I, Pfeilschifter J, Meng W et al. and the Basedow Study Group. Relapse of Graves' disease after successful outcome of antithyroid drug therapy: results of a prospective randomized study on the use of levothyroxine. Thyroid 2002;12(12):1119‐27. - PubMed
-
- Quadbeck B, Hoermann R, Janssen OE, Mann K. Drug treatment of immune hyperthyroidism (Basedow disease). Patient selection, long‐term follow‐up and prevention of recurrence [Medikamentose Behandliung der Immunhyperthyreose (Typ Morbus Basedow) Patientselektion, Langzeitverlauf und Rezidivprophylaxe]. Internist 2003;44:440‐8. - PubMed
Huszno 2004 {published data only}
-
- Huszno B, Trofimiuk M, Golkowski F, Plinta T, Szybinski Z. The value of early immunosuppressive therapy in the prevention of Graves' disease complications [Ocena wczesnego zastosowania leczenia immunosupresyjego w zapobiegania powiklaniom choroby Graves‐Basedowa]. Przeglad Lekarski 2004;61(8):868‐71. - PubMed
Jorde 1995 {published data only}
-
- Jorde R, Ytre‐Arne K, Stormer J, Sundsfjord J. Short‐term treatment of Graves' disease with methimazole in high versus low doses. Journal of Internal Medicine 1995;238(2):161‐5. - PubMed
Leclere 1994 {published data only}
-
- Leclere J. Treatment of Basedow disease with synthetic antithyroid drugs. Evaluation of the dose on the efficacy of the long term treatment [Traitement de la maladie de Basedow par anti‐thyrodiens de synthese. Evaluation de la dose sur l'efficacite du traitement a long terme (French)]. Annales d'Endocrinologie 1994;55(1):11‐4. - PubMed
Lucas 1997 {published data only}
-
- Lucas A, Salinas I, Rius F, Pizarro E, Granada ML, Foz M, et al. Medical therapy of Graves' disease: does thyroxine prevent recurrence of hyperthyroidism?. Journal of Clinical Endocrinology and Metabolism 1997;82(8):2410‐3. [MEDLINE: ; CN‐00142419] - PubMed
Mastorakos 2003 {published data only}
-
- Mastorakos G, Doufas AG, Mantoz E, Mantoz J, Koutras DA. T4 but not T3 administration is associated with increased recurrence of Graves' disease after successful medical therapy. Journal of Endocrinological Investigation 2003;26:979‐984. - PubMed
Maugendre 1999 {published data only}
-
- Maugendre D, Gatel A, Campion L, Massart C, Guilhem I, Lorcy Y, et al. Antithyroid drugs and Graves' disease ‐ prospective randomized assessment of long‐term treatment [Antithyroid drugs and Graves' disease ‐ prospective randomized assessment of long‐term treatment]. Clinical Endocrinology 1999;50(1):127‐32. - PubMed
McIver B 1996 {published data only}
-
- McIver B, Rae P, Beckett G, Wilkinson E, Gold A, Toft A. Lack of effect of thyroxine in patients with Graves' hyperthyroidism who are treated with an antithyroid drug [Lack of effect of thyroxine in patients with Graves' hyperthyroidism who are treated with an antithyroid drug]. New England Journal of Medicine 1996;334(4):220‐4. - PubMed
Nedrebo 2002 {unpublished data only}
-
- Nedrebo BG, Holm P, Uhlving S, Sorheim JI, Skeie S, Eide GE, et al. Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves' disease [Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves' disease]. European Journal of Endocrinology 2002;147(5):583‐9. - PubMed
Peixoto 2006 {published data only}
-
- Peixoto MC, Buescu A, Goncalves MRB, Albernaz MDS, Coeli CM, Vaisman M. Antithyroid drugs for the treatment of graves disease: A randomized clinical trial. Endocrinologist 2006;16(6):344‐8.
Pfeilschifter 1997 {published data only}
-
- Pfeilschifter J, Zeigler R. Suppression of serum thyrotropin with thyroxine in patients with Graves' disease: effects on recurrence of hyperthyroidism and thyroid volume [Suppression of serum thyrotropin with thyroxine in patients with Graves' disease: effects on recurrence of hyperthyroidism and thyroid volume]. European Journal of Endocrinology 1997;136(1):81‐6. - PubMed
Raber 2000 {published data only}
-
- Raber W, Kmen E, Waldhausl W, Vierhapper H. Medical therapy of Graves' disease: effect on remission rates of methimazole alone and in combination with triiodothyronine [Medical therapy of Graves' disease: effect on remission rates of methimazole alone and in combination with triiodothyronine]. European Journal of Endocrinology 2000;142(2):117‐24. - PubMed
Rittmaster 1998 {published data only}
-
- Rittmaster RS, Abbot EC, Douglas R, Givner ML, Lehman L, Reddy S, et al. Effect of methimazole, with or without L‐thyroxine, on remission rates in Graves' disease. Journal of Clinical Endocrinology and Metabolism 1998;83:814‐8. - PubMed
Tuncel 2000 {unpublished data only}
-
- Tuncel E, Ertturk E, Imamoglu S. Effects of antithyroid drugs alone and plus L‐thyroxine on the relapse of Graves disease [Effects of antithyroid drugs alone and plus L‐thyroxine on the relapse of Graves disease]. Journal of Endocrinological Investigation 2000;23(7Suppl):A71.
Weetman 1994 {published data only}
-
- Weetman AP, Pickerill AP, Watson P, Chatterjee VK, Edwards OM. Treatment of Graves' disease with the block‐replace regimen of antithyroid drugs: the effect of treatment duration and immunogenetic susceptibility on relapse. Quarterly Journal of Medicine 1994;87(6):337‐41. - PubMed
Wilson 1996 {published data only}
-
- Wilson R, Buchanan L, Fraser WD, McKillop JH, Thomson JA. Do higher doses of carbimazole improve remission in Graves' disease? [Do higher doses of carbimazole improve remission in Graves' disease?]. Quarterly Journal of Medicine 1996;89(5):381‐5.
References to studies excluded from this review
Duprey 1988 {published data only}
-
- Duprey J, Louis MF, Ducornet B, Lifchitz E, Mosse A, Sultan M, et al. Improvement of the prognosis of Basedow's disease by using high doses of carbimazole [Amelioratio du pronostic de la maladie de Basedow par utilisation de fortes doses de carbimazole (French)]. Presse Medicale 1988;17:1124‐7. - PubMed
El Fassi 2007 {published data only}
-
- Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedus L. B lymphocyte depletion with the monoclonal antibody rituximab in graves' disease: A controlled pilot study.. Journal of Clinical Endocrinology and Metabolism 2007;92(5):1769‐1772. - PubMed
Escobar‐Morreale 96 {published data only}
-
- Escobar‐Morreale HF, Serrano‐Gotarredona J, Villar LM, Garcia‐Robles R, Gonzalez‐Porque P, Sancho JM, et al. Methimazole has no dose‐related effect on the serum concentrations of soluble class I major histocompatibility complex antigens, soluble interleukin‐2 receptor, and beta 2‐microglobulin in patients with Graves' disease [Methimazole has no dose‐related effect on the serum concentrations of soluble class I major histocompatibility complex antigens, soluble interleukin‐2 receptor, and beta 2‐microglobulin in patients with Graves' disease]. Thyroid 1996;6(1):29‐36. - PubMed
Gwinup 1978 {published data only}
-
- Gwinup G. Prospective randomized comparison of propylthiouracil [Prospective randomized comparison of propylthiouracil]. Journal of the American Medical Association 1978;239(23):2457‐9. - PubMed
Hagag 1998 {published data only}
-
- Hagag P, Nissenbaum H, Weiss M. Role of colestipol in the treatment of hyperthyroidism [Role of colestipol in the treatment of hyperthyroidism]. Journal of Endocrinological Investigation 1998;21:725‐31. - PubMed
He 2004 {published data only}
-
- He CT, Hsieh AT, Pei D, Hung YJ, Wu LY, Yang TC, Lian WC, Huang WS, Kuo SW. Comparison of single daily dose of methimazole and propylthiouracil in the treatment of Graves' hyperthyroidism. Clinical endocrinology 2004;60(6):676‐81. - PubMed
Heemstra 2008 {published data only}
-
- Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, Romijn JA, Smith JW. Rituximab in relapsing Graves' disease, a phase II study. European Journal of Endocrinology 2008;159(5):609‐615. - PubMed
Homsanit 2001 {published data only}
-
- Homsanit M, Sriussadaporn S, Vannasaeng S, Peerapatdit T. Efficacy of single daily dosage of methimazole vs. propylthiouracil in the induction of euthyroidism [Efficacy of single daily dosage of methimazole vs. propylthiouracil in the induction of euthyroidism]. Clinical Endocrinology 2001;54:385‐90. - PubMed
Kallner 1996 {published data only}
-
- Kallner G, Vitols S, Ljunggren JG. Comparison of standardized initial doses of two antithyroid drugs in the treatment of Graves' disease [Comparison of standardized initial doses of two antithyroid drugs in the treatment of Graves' disease]. Journal of Internal Medicine 1996;239:525‐9. - PubMed
Kristensen 1976 {published data only}
-
- Kristensen O, Andersen HH, Pallisgaard G. Lithium carbonate in the treatment of thyrotoxicosis. A controlled trial [Lithium carbonate in the treatment of thyrotoxicosis. A controlled trial]. The Lancet 1976;1(7960):603‐5. - PubMed
Kubota 2005 {published data only}
-
- Kubota S, Ohye H, Nishihara E, Kudo T, Ito M, Fukata S, et al. Effect of high dose methylprednisolone pulse therapy followed by oral prednisolone administration on the production of anti‐TSH receptor antibodies and clinical outcome in Graves' disease. Endocrine Journal 2005;52(6):735‐741. - PubMed
Mashio 1988 {published data only}
-
- Mashio Y, Beniko M, Ikota A, Mizumoto H, Kunita H. Treatment of hyperthyroidism with a small single daily dose of methimazole [Treatment of hyperthyroidism with a small single daily dose of methimazole]. Acta Endocrinologica 1988;119:139‐44. - PubMed
Meng 1991 {published data only}
-
- Meng W, Meng S, Mannchen E, Hampel R, Kirsch G, Dannenberg J, et al. Effect of therapy duration and low and highly dosed thiamazole treatment in Basedow's‐Graves' disease. Experimental Clinical Endocrinology 1991;97(2‐3):257‐60. - PubMed
Messina 1987 {published data only}
-
- Messina M, Milani P, Gentile L, Monaco A, Brossa C, Porta M, et al. Initial treatment of thyrotoxic Graves' disease with methimazole: a randomized trial comparing different dosages [Initial treatment of thyrotoxic Graves' disease with methimazole: a randomized trial comparing different dosages]. Journal of Endocrinological Investigation 1987;10:291‐5. - PubMed
Nakamura 2007 {published data only}
-
- Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves' disease. Journal of Clinical Endocrinology and Metabolism 2007;92(6):2157‐62. - PubMed
Nicholas 1995 {published data only}
-
- Nicholas WC, Fischer RG, Stevenson RA. Single daily dose of methimazole compared to every 8 hours propylthiouracil in the treatment of hyperthyroidism. Southern Medical Journal 1995;88(9):973‐6. - PubMed
O'Malley 1988 {published data only}
-
- O'Malley BP, Rosenthal FD, Northover B, Woods KL. Higher than conventional doses of carbimazole in the treatment of thyrotoxicosis [Higher than conventional doses of carbimazole in the treatment of thyrotoxicosis]. Clinical Endocrinology 1988;29:281‐8. - PubMed
Page 1996 {published data only}
-
- Page SR, Sheard CE, Herbert M, Hopton M, Jeffcoate WJ. A comparison of 20 or 40 mg per day of carbimazole in the initial treatment of hyperthyroidism [A comparison of 20 or 40 mg per day of carbimazole in the initial treatment of hyperthyroidism]. Clinical Endocrinology 1996;45:511‐5. - PubMed
Pujol 1998 {published data only}
-
- Pujol P, Osman A, Grabar S, Daures JP, Galtier‐Dereure F, Baldet L, et al. TSH suppression combined with carbimazole for Graves' disease: effect on remission and relapse rates [TSH suppression combined with carbimazole for Graves' disease: effect on remission and relapse rates]. Clinical Endocrinology 1998;48(5):635‐40. - PubMed
Romaldini 1983 {published data only}
-
- Romaldini JH, Bromberg N, Werner RS, Tanaka LM, Rodrigues HF, Werner MC, et al. Comparison of effects of high and low dosage regimens of antithyroid drugs in the management of Graves' hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 1983;57(3):563‐70. [MEDLINE: ; CN‐00031731] - PubMed
Salvi 2007 {published data only}
-
- Salvi M, Vannucchi G, Campi I, Curro N, Dazzi D, Simonetta S, et al. Treatment of graves' disease and associated opthalmopathy with the anti‐CD20 monoclonal antibody rituximab: An open study. European Journal of Endocrinology 2007;156(1):33‐40. - PubMed
Soltani 2007 {published data only}
-
- Soltani A, Moayyeri A, Azizi F. Combination therapy of chloroquine and methimazole in Graves' disease: a pilot randomized controlled trial. Biomedicine & pharmacotherapy 2007;61(4):241‐3. - PubMed
Tamai 1995 {published data only}
-
- Tamai H, Hayaki I, Kawai K, Komaki G, Matsubayashi S, Kuma K, et al. Lack of effect of thyroxine administration on elevated thyroid stimulating hormone receptor antibody levels in treated Graves' disease patients [Lack of effect of thyroxine administration on elevated thyroid stimulating hormone receptor antibody levels in treated Graves' disease patients]. Journal of Clinical Endocrinology and Metabolism 1995;80(5):1481‐4. - PubMed
Tsai 2005 {published data only}
-
- Tsai WC, Pei D, Wang TF, Wu DA, Li JC, Wei CL, Lee CH, Chen SP, Kuo SW. The effect of combination therapy with propylthiouracil and cholestyramine in the treatment of Graves' hyperthyroidism. Clinical Endocrinology 2005;62(5):521‐4. - PubMed
Vrca 2004 {published data only}
-
- Vrca VB, Skreb F, Cepelak I, Romic Z, Mayer L. Supplementation with antioxidants in the treatment of Graves' disease; the effect on glutathione peroxidase activity and concentration of selenium. Clinica Chimica Acta 2004;341:55‐63. - PubMed
Weetman 1986 {published data only}
-
- Weetman AP, Ratanachaiyavong S, Middleton W, Lowe W, John R, Owen GM, et al. Prediction of outcome in Graves' disease after carbimazole treatment [Prediction of outcome in Graves' disease after carbimazole treatment]. Quarterly Journal of Medicine 1986;59(229):409‐19. - PubMed
Additional references
Cohen 1960
-
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46.
Cooper 2005
-
- Cooper D. Antithyroid drugs. New England Journal of Medicine 2005;352:905‐17. - PubMed
Dickersin 1994
Franklyn 1994
-
- Franklyn JA. The management of hyperthyroidism. New England Journal of Medicine 1994;330(24):1731‐8. - PubMed
Higgins 2003
Leech 1998
-
- Leech NJ, Dayan CM. Controversies in the management of Graves' disease. Clinical Endocrinology 1998;49(3):273‐80. - PubMed
Toft 1997
-
- Toft A. Thyroxine suppression therapy in Graves' disease [Thyroxine suppression therapy in Graves' disease]. Bailliere's Clinical Endocrinology and Metabolism 1997;11(3):537‐48. - PubMed
References to other published versions of this review
Abraham 2005
-
- Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. European Journal of Endocrinology 2005;153:489‐98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

