Interleukin 2 receptor antagonists for kidney transplant recipients
- PMID: 20091551
- PMCID: PMC7154335
- DOI: 10.1002/14651858.CD003897.pub3
Interleukin 2 receptor antagonists for kidney transplant recipients
Abstract
Background: Interleukin 2 receptor antagonists (IL2Ra) are used as induction therapy for prophylaxis against acute rejection in kidney transplant recipients. Use of IL2Ra has increased steadily since their introduction, but the proportion of new transplant recipients receiving IL2Ra differs around the globe, with 27% of new kidney transplant recipients in the United States, and 70% in Australasia receiving IL2Ra in 2007.
Objectives: To systematically identify and summarise the effects of using an IL2Ra, as an addition to standard therapy, or as an alternative to another immunosuppressive induction strategy.
Search strategy: We searched the Cochrane Renal Group's specialised register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE to identify new records, and authors of included reports were contacted for clarification where necessary.
Selection criteria: Randomised controlled trials (RCTs) in all languages comparing IL2Ra to placebo, no treatment, other IL2Ra or other antibody therapy.
Data collection and analysis: Data was extracted and assessed independently by two authors, with differences resolved by discussion. Dichotomous outcomes are reported as relative risk (RR) and continuous outcomes as mean difference (MD) with 95% confidence intervals (CI).
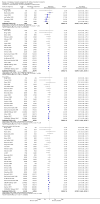
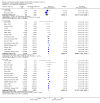
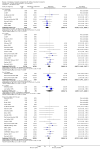
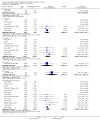
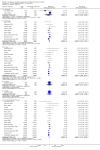
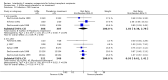
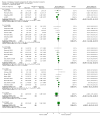
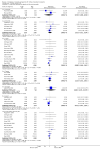
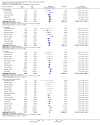
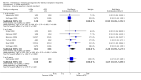
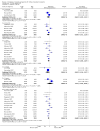
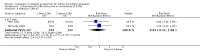
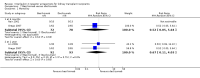
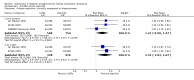
Main results: We included 71 studies (306 reports, 10,537 participants). Where IL2Ra were compared with placebo (32 studies; 5,784 patients) graft loss including death with a functioning graft was reduced by 25% at six months (16 studies: RR 0.75, 95% CI 0.58 to 0.98) and one year (24 studies: RR 0.75, 95% CI 0.62 to 0.90), but not beyond this. At one year biopsy-proven acute rejection was reduced by 28% (14 studies: RR 0.72, 95% CI 0.64 to 0.81), and there was a 19% reduction in CMV disease (13 studies: RR 0.81, 95% CI 0.68 to 0.97). There was a 64% reduction in early malignancy within six months (8 studies: RR 0.36, 95% CI 0.15 to 0.86), and creatinine was lower (7 studies: MD -8.18 micromol/L 95% CI -14.28 to -2.09) but these differences were not sustained.When IL2Ra were compared to ATG (16 studies, 2211 participants), there was no difference in graft loss at any time point, or for acute rejection diagnosed clinically, but the was benefit of ATG therapy over IL2Ra for biopsy-proven acute rejection at one year (8 studies:, RR 1.30 95% CI 1.01 to 1.67), but at the cost of a 75% increase in malignancy (7 studies: RR 0.25 95% CI 0.07 to 0.87) and a 32% increase in CMV disease (13 studies: RR 0.68 95% CI 0.50 to 0.93). Serum creatinine was significantly lower for IL2Ra treated patients at six months (4 studies: MD -11.20 micromol/L 95% CI -19.94 to -2.09). ATG patients experienced significantly more fever, cytokine release syndrome and other adverse reactions to drug administration and more leucopenia but not thrombocytopenia. There were no significant differences in outcomes according to cyclosporine or tacrolimus use, azathioprine or mycophenolate, or to the study populations baseline risk for acute rejection. There was no evidence that effects were different according to whether equine or rabbit ATG was used.
Authors' conclusions: Given a 38% risk of rejection, per 100 recipients compared with no treatment, nine recipients would need treatment with IL2Ra to prevent one recipient having rejection, 42 to prevent one graft loss, and 38 to prevent one having CMV disease over the first year post-transplantation. Compared with ATG treatment, ATG may prevent some experiencing acute rejection, but 16 recipients would need IL2Ra to prevent one having CMV, but 58 would need IL2Ra to prevent one having malignancy. There are no apparent differences between basiliximab and daclizumab. IL2Ra are as effective as other antibody therapies and with significantly fewer side effects.
Conflict of interest statement
Dr Jeremy Chapman: has advisory board and clinical trial involvement with Novartis, Roche, Janssen‐Cilag, Fujisawa and Wyeth, and has also been an invited speaker at national and international meetings sponsored by these companies.
ACW, JCC, NW, GYH, LPR, RMG, SLM ‐ none declared
Figures




























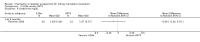
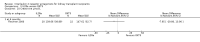




















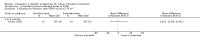







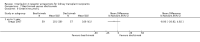




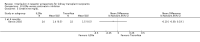
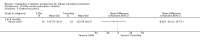








Update of
-
Interleukin 2 receptor antagonists for kidney transplant recipients.Cochrane Database Syst Rev. 2004;(1):CD003897. doi: 10.1002/14651858.CD003897.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003897. doi: 10.1002/14651858.CD003897.pub3. PMID: 14974043 Updated.
References
References to studies included in this review
Abou‐Ayache 2008 {published data only}
-
- Abou‐Ayache R, Buchler M, Lepogamp P, Westeel PF, Meur Y, Etienne I, et al. CMV infections after two doses of daclizumab versus thymoglobulin in renal transplant patients receiving mycophenolate mofetil, steroids and delayed cyclosporine A. Nephrology Dialysis Transplantation 2008;23(6):2024‐32. [MEDLINE: ] - PubMed
-
- Abou‐Ayache R, Lebranchu Y, Leopgamp P, Westeel J, Meur Y, Etienne I, et al. Two‐dose daclizumab induction treatment versus thymoglobuline in renal transplant patients receiving a mycophenolate mofetil based immunosuppression [abstract no: 121]. American Journal of Transplantation 2005;5(Suppl 11):187. [CENTRAL: CN‐00644200]
Ahsan 2002 {published data only}
-
- Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC. Limited dose monoclonal IL‐2R antibody induction protocol after primary kidney transplantation. American Journal of Transplantation 2002;2(6):568‐73. [MEDLINE: ] - PubMed
-
- Ahsan N, Holman MJ, Yang HC. Limited dose monoclonal IL‐2R antibody induction in kidney transplantation ‐ a prospective, randomized, controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):469. [CENTRAL: CN‐00400019] - PubMed
Asberg 2006 {published data only}
-
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR‐matched de novo kidney transplant recipients. Transplantation 2006;82(1):62‐8. [MEDLINE: ] - PubMed
-
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor‐avoidance with daclizumab, mycophenolate mofetil and prednisolone in DR matched de novo kidney transplant recipients [abstract no: SP737]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv264. - PubMed
ATLAS 2003 {published data only}
-
- Klinger M, Vitko S, Salmela K, Wlodarczyk Z, Tyden G, the ATLAS Study Group. Large, prospective study evaluating steroid‐free immunosuppression with tacrolimus/basiliximab and tacrolimus/mmf compared with tacrolimus/mmf/steroids in renal transplantation [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788‐9. [CENTRAL: CN‐00446121]
-
- Kramer BK, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Vitko S. Two steroid‐free immunosuppressive regimens (basiliximab/tacrolimus and tacrolimus/mmf) in comparison to tacrolimus/MMF/steroid therapy after renal transplantation [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):9A. [CENTRAL: CN‐00583329]
-
- Kramer BK, Kruger B, Hoffmann U, Wlodarczyk Z, Tyden G, Senatorski G, et al. 1‐year‐follow‐up of two steroid‐free immunosuppressive regimens ‐ basiliximab/tacrolimus and tacrolimus/MMR ‐ in comparison to tacrolimus/MMF/steroids after renal transplantation [abstract no: F‐PO1026]. Journal of the American Society of Nephrology 2004;15(Oct):289A. [CENTRAL: CN‐0058338]
-
- Kramer BK, Kruger B, MacK M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: Focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐91. [MEDLINE: ] - PubMed
-
- Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, et al. Two corticosteroids‐free regimens ‐ tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil ‐ in comparison with a standard triple regimen in renal transplantation: results of the Atlas Study. Transplantation 2005;80(12):1734‐41. [MEDLINE: ] - PubMed
Baczkowska 2002 {published data only}
-
- Baczkowska T, Durlik M, Perkowska A, Sadowska A, Cieciura T, Nowacka‐Cieciura E, et al. [Cytokines and growth factors serum level and renal allograft function (preliminary report)] [Polish]. Polski Merkuriusz Lekarski 2003;15(88):356‐8. [MEDLINE: ] - PubMed
-
- Baczkowska T, Perkowska A, Cieciura T, Wierzbicki P, Klosowka D, Matlosz B, et al. Daclizumab allows for a protocol with low‐dose cyclosporine in low rejection‐risk kidney recipients ‐ preliminary data [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):309. [CENTRAL: CN‐00400168]
-
- Baczkowska T, Perkowska‐Francka A, Durlik M, Cieciura T, Nowacka‐Cieciura E, Pazik J, et al. The role of the protocol biopsies in renal allograft recipients. Transplantation Proceedings 2003;35(6):2179‐81. [MEDLINE: ] - PubMed
-
- Baczkowska T, Perkowska‐Ptasinska A, Cieciura T, Pazik J, Nowacka‐Cieciura E, Lewandowski Z, et al. Untreated subclinical, borderline rejection in 12 and 36‐month protocol biopsies is not associated with progressive loss of GFR at 5‐year's follow‐up [abstract no: 1755]. Transplantation 2008;86(Suppl 2):581. [CENTRAL: CN‐00671803]
-
- Baczkowska T, Perkowska‐Ptasinska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, Pazik J, et al. Serum TGF‐b1 correlates with chronic histopathological lesions in protocol biopsies in kidney allograft recipients [abstract]. Transplantation. 2004;78(2 Suppl):319. [CENTRAL: CN‐00583398] - PubMed
Bernarde 2004 {published data only}
-
- Bernarde K, Folkmane I, Rozentals R, Bicans J. Induction immunosuppression with interleukin‐2 receptor antibodies (basiliximab) in renal transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):467. [CENTRAL: CN‐00509086]
Bingyi 2003 {published data only}
-
- Bingyi S, Ming C, Yeyong Q, Chunbai M, Wenqiang Z. The effect of anti‐CD25 monoclonal antibody (Simulect) to the lymphocytes in the peripheral blood of the recipients of kidney transplantation. Transplantation Proceedings 2003;35(1):243‐5. [MEDLINE: ] - PubMed
-
- Bingyi S, Yeyong Q, Ming C, Chunbai M, Wenqiang Z. Randomised trial of Simulect versus placebo for control of acute rejection in renal allograft recipients. Transplantation Proceedings 2003;35(1):192‐4. [MEDLINE: ] - PubMed
Brennan 2006 {published and unpublished data}
-
- Brennan DC, Daller JA, Lake KD, Cibrik D, Castillo D, Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. New England Journal of Medicine 2006;355(19):1967‐77. [MEDLINE: ] - PubMed
-
- Brennan DC, Schnitzler M. 5 Year outcomes in a randomized trial comparing rabbit antithymocyte globulin and basiliximab in kidney transplant recipients: Linking clinical trial data with registry data: 798. [Abstract]. Transplantation 2008;86(2 Suppl):279.
-
- Brennan DC, Schnitzler MA. Long‐Term results of rabbit antithymocyte globulin and basiliximab induction. New England Journal of Medicine 2008;359(16):1736‐8. [MEDLINE: ] - PubMed
-
- Brennan DC, Thymoglobulin Induction Study Group. A prospective randomized multicenter comparison of thymoglobulin versus Simulect for induction therapy in high risk renal transplant recipients [abstract no:398]. American Journal of Transplantation 2002;2(Suppl 3):238. [CENTRAL: CN‐00400376]
-
- Brennan DC, Thymoglobulin Induction Study Group. Thymoglobulin versus Simulect for induction immunosuppression in cadaveric renal transplant recipients: expanded results from a prospective, randomized, multicenter trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):438‐9. [CENTRAL: CN‐00444533]
CAESAR (Ekberg) 2007 {published data only}
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, CAESAR Study Group. Low‐dose cyclosporine in conjunction with daclizumab, mycophenolate mofetil and corticosteroids is safe and effective in contrast to early cyclosporine withdrawal [abstract]. Transplantation 2004;78(Suppl 2):458.
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Calleja E, et al. The use of daclizumab and mycophenolate mofetil in combination with corticosteroids and cyclosporine (low dose versus low dose followed by withdrawal) to optimize renal function in recipients of renal allografts [abstract]. Transplantation 2004;78(2 Suppl):458. [CENTRAL: CN‐00509171]
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. American Journal of Transplantation 2007;7(3):560‐70. [MEDLINE: ] - PubMed
-
- Grinyo J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Spleiss O, Rashford M, Nasmyth‐Miller C, Essioux L. Association of three polymorphisms with acute kidney transplantation: an exploratory pharmacogenetic analysis of a randomized mulitcenter clinical trial (The CAESaR study). American Journal of Transplantation 2006;6(Suppl 2):410.
-
- Vincenti F, Vanrenterghem Y, Nashan B, Grinyo J, Ekberg H, Nasmyth‐Miller C, et al. The use of mycophenolate mofetil, daclizumab and corticosteroids with cyclosporine (low dose, low dose/withdrawal and standard dose) to optimize renal function in renal allograft recipients ‐ 18 month results [abstract no: 1507]. American Journal of Transplantation 2005;5(Suppl 11):539. [CENTRAL: CN‐00644286]
CARMEN (Rostaing) 2005 {published data only}
-
- Budde K, Neumayer HH, Rostaing L, Catarovich D, Mourad G, Rigotti P, et al. Steroid‐free immunosuppression with daclizumab, tacrolimus and mmf is efficacious and improves cholesterol, glucose and bone mineral density ‐ the CARMEN study [abstract]. Transplantation 2004;78(2 Suppl):168. [CENTRAL: CN‐00509111]
-
- Cantarovich D, Rostaing L, Mourad G, Neumayer HH, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. The combination of daclizumab, tacrolimus, and MMF is an effective and safe steroid‐free immunosuppressive regimen after renal transplantation. Results of a large multicentre trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788. [CENTRAL: CN‐00444672]
-
- Kramer BK, Kruger B, Mack M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: Focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐1791. [MEDLINE: ] - PubMed
-
- Mourad GL, Rostaing D, Cantarovich H, Neumayer H, Rigotti P, the Tacrolimus Steroidfree Study Group. Immunosuppression without steroids: daclizumab/tacrolimus/MMF vs. tacrolimus/MMF/steroids in renal transplantation [abstract no: 12]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003.
-
- Pascual J, Rigotti P, Vialtel P, Sanchez‐Rructuoso A, Escuin F, The Bone Density Study Group. Immunosuppression without steroids: a daclizumab, tacrolimus and MMF regimen prevents loss of bone mass following renal transplantation [abstract no 369]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003.
Cerrillos 2006 {published data only}
-
- Cerrillos I, Gomez‐Navarro B, Cueto A, Ramos F, Monteon F. Daclizumab two doses 0 and 4 days is efficacious to prevent rejection after kidney transplantation [abstract no: PUB163]. Journal of the American Society of Nephrology 2006;17(Abstracts):850A. [CENTRAL: CN‐00615829]
Chen 2003 {published data only}
-
- Chen J, Huang H, Peng W, Wu J. Double filtration plasmapheresis with/without daclizumab induction in the sensitized candidates of cadaveric renal transplantation: a randomized prospective trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):494. [CENTRAL: CN‐00444777]
-
- Chen J, Peng W, Wu J. The effect of daclizumab in highly sensitive kidney recipients [abstract no: SA‐PO652]. Journal of the American Society of Nephrology 2003;14(Nov):440A. [CENTRAL: CN‐00583413]
Ciancio 2005 {published data only}
-
- Carreno MR, Ciancio G, Burke GW, Rosen A, Ricordi C, Tzakis A, et al. Cellular phenotypes affected by induction therapy with campath‐1h vs thymoglobulin vs Zenapax in kidney allograft recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):405. [CENTRAL: CN‐00509121]
-
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune‐monitoring. Transplantation 2005;80(4):457‐65. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi AD, Carreno MR, Rosen A, et al. Randomized trial of three different induction regimens to prevent acute renal allograft rejection: early results [abstract]. American Journal of Transplantation 2004;4(Suppl 8):266.
-
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow‐up. Clinical Transplantation 2008;22(2):200‐10. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Mattiazzi A, Illanes HG, Gaynor JJ, Carreno MR, et al. A randomized trial of three different antibody induction regimens in renal transplantation. American Journal of Transplantation 2005;5(Suppl 11):569. [CENTRAL: CN‐00644195]
Clatworthy 2009 {published data only}
Dac double & triple {published data only}
-
- See Dacilizumab Double and Triple studies.
Daclizumab double 1999 {published data only}
-
- Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3‐year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation 2001;72(5):839‐45. [MEDLINE: ] - PubMed
-
- Bumgardner GL, Ramos E, Lin A, Vincenti F, Daclizumab Triple Therapy and Double Therapy Groups. Daclizumab (humanized anti‐IL2R alpha mAb) prophylaxis for prevention of acute rejection in renal transplant recipients with delayed graft function. Transplantation 2001;72(4):642‐7. [MEDLINE: ] - PubMed
-
- Charpentier B, Thervet E. Placebo‐controlled study of a humanized anti‐TAC monoclonal antibody in dual therapy for prevention of acute rejection after renal transplantation. Transplantation Proceedings 1998;30(4):1331‐2. [MEDLINE: ] - PubMed
-
- Ekberg H, Backman L, Tufveson G, Tyden G. Zenapax (daclizumab) reduces the incidence of acute rejection episodes and improves patient survival following renal transplantation. No 14874 and No 14393 Zenapax Study Groups. Transplantation Proceedings 1999;31(1‐2):267‐8. [MEDLINE: ] - PubMed
-
- Ekberg H, Backman L, Tufveson G, Tyden G, Nashan B, Vincenti F. Daclizumab prevents acute rejection and improves patient survival post transplantation: 1 year pooled analysis. Transplant International 2000;13(2):151‐9. [MEDLINE: ] - PubMed
Daclizumab triple 1998 {published data only}
-
- Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3‐year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation 2001;72(5):839‐45. [MEDLINE: ] - PubMed
-
- Bumgardner GL, Ramos E, Lin A, Vincenti F, Daclizumab Triple Therapy and Double Therapy Groups. Daclizumab (humanized anti‐IL2Ralpha mAb) prophylaxis for prevention of acute rejection in renal transplant recipients with delayed graft function. Transplantation 2001;72(4):642‐7. [MEDLINE: ] - PubMed
-
- Ekberg H, Backman L, Tufveson G, Tyden G. Zenapax (daclizumab) reduces the incidence of acute rejection episodes and improves patient survival following renal transplantation. No 14874 and No 14393 Zenapax Study Groups. Transplantation Proceedings 1999;31(1‐2):267‐8. [MEDLINE: ] - PubMed
-
- Ekberg H, Backman L, Tufveson G, Tyden G, Nashan B, Vincenti F. Daclizumab prevents acute rejection and improves patient survival post transplantation: 1 year pooled analysis. Transplant International 2000;13(2):151‐9. [MEDLINE: ] - PubMed
-
- Ekberg H, Backman L, Tufveson G, Tyden G, on behalf of the NO 14874 and NO 14393 Zenapax Study Groups. Daclizumab (Zenapax) reduces the incidence of acute rejection episodes following renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00400813]
de Boccardo 2002 {published data only}
-
- Boccardo G. Latin American study of the efficacy and safety of Simulect in kidney transplant recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00400671]
Fangmann 2004 {published data only}
-
- Fangmann J, Arns W, Marti H, Budde K, Beckurts T, Hauss J. Low dose cyclosporine regimen with daclizumab induction and mycophenolate mofetil after kidney transplantation ‐ impact on renal function and rejection episodes [abstract no: 113]. American Journal of Transplantation 2005;5(Suppl 11):185. [CENTRAL: CN‐00644197]
-
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):353. [CENTRAL: CN‐00509182]
-
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):280.
Flechner 2000 {published data only}
-
- Flechner SM, Goldfarb DA, Fairchild R, Cook D, Mastroianni B, Fisher R, et al. A randomized prospective trial of OKT3 vs basiliximab for induction therapy in renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S157. [CENTRAL: CN‐00400926] - PubMed
Folkmane 2001 {published data only}
-
- Folkmane I, Bicans J, Amerika D, Chapenko S, Murovska M, Rosentals R. Low rate of acute rejection and cytomegalovirus infection in kidney transplant recipients with basiliximab. Transplantation Proceedings 2001;33(7‐8):3209‐10. [MEDLINE: ] - PubMed
-
- Folkmane I, Bicans J, Chapenko S, Murovska M, Rosentals R. Results of renal transplantation with different immunosuppressive regimens. Transplantation Proceedings 2002;34(2):558‐9. [MEDLINE: ] - PubMed
-
- Folkmane I, Chapenko S, Murovska M, Rosental R. Low rate of acute rejection and cytomegalovirus infection in renal transplant recipients with basiliximab [abstract no:1037]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. - PubMed
Garcia 2002 {published data only}
-
- Garcia R, Hanzawa NM, Machado PGP, Moreira SR, Prismich G, Felipe CR, et al. A calcineurin inhibitor‐free regimen for low risk kidney transplant recipients [abstract no:2379]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
Gelens 2006 {published data only}
-
- Gelens M, Christiaans M, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
-
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] - PubMed
Grego 2007 {published data only}
-
- Grego K, Arnol M, Bren AF, Kmetec A, Tomazic J, Kandus A. Basiliximab versus daclizumab combined with triple immunosuppression in deceased donor renal graft recipients. Transplantation Proceedings 2007;39(10):3093‐7. [MEDLINE: ] - PubMed
-
- Grego K, Kandus A, Bren AF. Basiliximab versus daclizumab for prevention of acute renal allograft rejection [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2006;17(Abstracts):223A. [CENTRAL: CN‐00602013]
Grenda 2006 {published data only}
-
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J, Fitzpatrick M, et al. A prospective, randomized, multicenter trial of tacrolimus‐based therapy with or without basiliximab in pediatric renal transplantation. American Journal of Transplantation 2006;6(7):1666‐72. [MEDLINE: ] - PubMed
-
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J, Paediatric Tacrolimus Study Group. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomised study in paediatric kidney transplantation [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
-
- Vondrak K, Grenda R, Watson AR, Webb NJA, Beattie J, Pediatric Tacrolimus Study Group. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomized study in pediatric kidney transplantation [abstract no: 964]. American Journal of Transplantation 2005;5(Suppl 11):401.
-
- Webb N, Prokurat S, Vondrak K, Watson A, Hughes D, Hamer C, et al. Multicentre randomized prospective trial of tacrolimus, azathioprine and prednisolone with or without basiliximab; two year follow‐up data [abstract no: 121 (FC)]. Paediatric Nephrology 2007;22(9):1446. [CENTRAL: CN‐00653717] - PubMed
Hanaway 2008 {published data only}
-
- Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (Alemtuzumab, Thymoglobulin and Basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 135]. American Journal of Transplantation 2008;8(Suppl 2):215. [CENTRAL: CN‐00653740]
-
- Holman J, Harrison G, Vandeputte K, First R, Fitzsimmons W. Immune cell activation comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 553]. Transplantation 2008;86(2 Suppl):194. [CENTRAL: CN‐00676047]
-
- Woodle S, Hanaway M, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 876]. Transplantation 2008;86(2 Suppl):306. [CENTRAL: CN‐00653740]
Hernandez 2007 {published data only}
-
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] - PubMed
Hourmant 1994 {published data only}
-
- Hourmant M, Mauff B, Cantarovich D, Dantal J, Baatard R, Denis M, et al. Prevention of acute rejection episodes with an anti‐interleukin 2 receptor monoclonal antibody. II. Results after a second kidney transplantation. Transplantation 1994;57(2):204‐207. [MEDLINE: ] - PubMed
Ji 2007 {published data only}
-
- Ji SM, Li LS, Cheng Z, Cheng DR, Sun QQ, Chen JS, et al. A single‐dose daclizumab induction protocol in renal allograft recipients: a Chinese single center experience. Transplantation Proceedings 2007;39(5):1396‐401. [MEDLINE: ] - PubMed
Kahan 1999 {published data only}
-
- Hall M, Kovarik J, Gerbeau C, Schmidt AG. Influence of the duration of IL‐2 receptor (IL‐2R) blockade on the incidence of acute rejection episodes in renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998.
-
- Kahan BD, Rajagopalan PR, Hall M, United States Simulect Renal Study Group. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti‐interleukin‐2‐receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation 1999;67(2):276‐284. [MEDLINE: ] - PubMed
-
- Kahan BD, Rajagopalan PR, Hall ML. Reduction of acute cellular rejection in renal allograft patients with basiliximab (Simulect). 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:260.
-
- Kahan BD, Rajagopalan PR, Hall ML, Kovarik JM, US Simulect Study Group. Basiliximab (Simulect) is efficacious in reducing the incidence of acute rejection episodes in renal allograft patients: results at 12 months [abstract]. Transplantation 1998;65(12):S189. [CENTRAL: CN‐00401446]
-
- Kahan BD, Rajagopalan PR, Hall ML, Kovarik JM, US Simulect Study Group. Basiliximab (Simulect) is efficacious in reducing the incidence of acute rejection episodes in renal allograft patients: results at 12 months [abstract]. Transplantation 1998;66(8):S1.
Kaplan 2003 {published data only}
-
- Kaplan B, Cibrik DM, Schold JD, Mulgaonkar S, Magee J, Howell T, et al. Pilot randomized prospective study of dual vs triple immunosuppression in older renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):212.
Khan 2000 {published data only}
-
- Khan AJ, Sarkissian N, Brennen TS, Gonzalez JM, Nassar GM, Achkar K, et al. Comparison of two IL‐2 receptor blockers in decreasing the incidence of acute rejection in early post‐transplant time in renal transplant recipients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):694A. [CENTRAL: CN‐00433633]
Kim 2008a {published data only}
-
- Kim MJ, Tsinalis D, Franz S, Binet I, Gurke L, Mihatsch MJ, et al. ATG‐Fresenius or daclizumab induction therapy in immunologically high risk kidney recipients: a prospective randomized pilot trial. Annals of Transplantation 2008;13(4):21‐7. [MEDLINE: ] - PubMed
Kirkman 1989 {published data only}
-
- Carpenter CB, Kirkman RL, Shapiro ME, Milford EL, Tiney NL, Waldmann TA, et al. Prophylactic use of monoclonal anti‐IL‐2 receptor antibody in cadaveric renal transplantation. American Journal of Kidney Diseases 1989;14(5 Suppl 2):54‐7. [MEDLINE: ] - PubMed
-
- Kirkman RL, Shapiro ME, Carpenter CB, Milford EL, Ramos EL, Tilney NL, et al. Early experience with anti‐Tac in clinical renal transplantation. Transplantation Proceedings 1989;21(1 Pt 2):1766‐8. [MEDLINE: ] - PubMed
-
- Ramos EL, Leggat JE, Milford EL, Kirkman RL, Tilney NL, Strom TB, et al. In vivo anti‐interleukin‐2 receptor (anti‐Tac) therapy is immunosuppressive, but not tolerogenic. Transactions of the Association of American Physicians 1989;102:231‐9. [MEDLINE: ] - PubMed
-
- Ramos EL, Milford EL, Kirkman RL, Tilney NL, Strom TB, Shapiro ME, et al. Differential IL‐2 receptor expression in renal allograft recipients treated with an anti‐IL‐2‐receptor antibody. Transplantation 1989;48(3):415‐20. [MEDLINE: ] - PubMed
Kirkman 1991 {published data only}
-
- Carpenter CB, Kirkman RL, Shapiro ME, Milford EL, Tiney NL, Waldmann TA, et al. Prophylactic use of monoclonal anti‐IL‐2 receptor antibody in cadaveric renal transplantation. American Journal of Kidney Diseases 1989;14(5 Suppl 2):54‐7. [MEDLINE: ] - PubMed
-
- Kirkman RL, Shapiro ME, Carpenter CB, McKay DB, Milford EL, Ramos EL, et al. A randomized prospective trial of anti‐Tac monoclonal antibody in human renal transplantation. Transplantation 1991;51(1):107‐13. [MEDLINE: ] - PubMed
-
- Kirkman RL, Shapiro ME, Carpenter CB, McKay DB, Milford EL, Ramos EL, et al. A randomized prospective trial of anti‐Tac monoclonal antibody in human renal transplantation. Transplantation Proceedings 1991;23(1 Pt 2):1066‐7. [MEDLINE: ] - PubMed
-
- Ramos EL, Leggat JE, Milford EL, Kirkman RL, Tilney NL, Strom TB, et al. In vivo anti‐interleukin‐2 receptor (anti‐Tac) therapy is immunosuppressive, but not tolerogenic. Transactions of the Association of American Physicians 1989;102:231‐9. [MEDLINE: ] - PubMed
-
- Ramos EL, Milford EL, Kirkman RL, Tilney NL, Strom TB, Shapiro ME, et al. Differential IL‐2 receptor expression in renal allograft recipients treated with an anti‐IL‐2‐receptor antibody. Transplantation 1989;48(3):415‐20. [MEDLINE: ] - PubMed
Kriaa 1993 {published data only}
-
- Beaudreuil S, Durrbach A, Noury J, Ducot B, Kriaa F, Bazin H, et al. Long‐term results (10 years) of a prospective trial comparing Lo‐tact‐1 monoclonal antibody and anti‐thymocyte globulin induction therapy in kidney transplantation. Transplant International 2006;19(10):814‐20. [MEDLINE: ] - PubMed
-
- Beaudreuil S, Durrbach A, Noury J, Kriaa F, Bazin H, Charpentier B. Long term follow‐up (10 years) of a prospective trial assay comparing lo‐tact‐1 antibody versus anti‐thymocyte globulin induction therapy in kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):467‐8. [CENTRAL: CN‐00509085] - PubMed
-
- Kriaa F, Hiesse C, Alard P, Lantz O, Noury J, Charpentier B, et al. Prophylactic use of the anti‐IL‐2 receptor monoclonal antibody LO‐Tact‐1 in cadaveric renal transplantation: results of a randomized study. Transplantation Proceedings 1993;25(1 Pt 1):817‐9. [MEDLINE: ] - PubMed
Kumar 2005 {published data only}
-
- Fa K, Kode RK, Lu Q, Kumar MSA, Laftavi MR, Pankewycz OG. Value of one month protocol biopsies combined with a molecular analysis in predicting efficacy of rapid steroid withdrawal after renal transplantation [abstract]. American Journal of Transplantation 2002;2(Suppl 3):171.
-
- Fa K, Laftavi MR, Ferry E, Kumar AMS, Fyfe B, Pankewycz OG. The predictive value of subclinical rejection in a steroid free immunosuppressive regimen [abstract]. American Journal of Transplantation 2003;3(Suppl 5):480.
-
- Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, et al. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 2006;81(6):832‐9. [MEDLINE: ] - PubMed
-
- Kumar MSA, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract no:2440]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00416079]
-
- Kumar MSA, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):393.
Kyllonen 2007 {published data only}
-
- Kyllonen L, Eklund B, Matinlauri I, Salmela K. Induction with single bolus ATG or basiliximab in cadaveric kidney transplantation with cyclosporin immunosuppression [abstract]. XIXth International Congress of the Transplantation Society, Miami, Florida. 2002 Aug 25‐30. [CENTRAL: CN‐00401573]
-
- Kyllonen L, Eklund B, Matinlauri I, Salmela K. Induction with single bolus ATGor basiliximab in cadaveric kidney transplantation with cyclosporin immunosuppression [abstract no: 2330]. Transplantation 2002;74(4 Suppl):466. [CENTRAL: CN‐00401573]
-
- Kyllonen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84(1):75‐82. [MEDLINE: ] - PubMed
-
- Matinlauri IH, Kyllonen LE, Eklund BH, Koskimies SA, Salmela KT. Weak humoral posttransplant alloresponse after a well‐HLA‐matched cadaveric kidney transplantation. Transplantation 2004;78(2):198‐204. [MEDLINE: ] - PubMed
-
- Matinlauri IH, Kyllonen LE, Salmela KT, Helin H, Pelzl S, Susal C. Serum sCD30 in monitoring of alloresponse in well HLA‐matched cadaveric kidney transplantations. Transplantation 2005;80(12):1809‐12. [MEDLINE: ] - PubMed
Lacha 2001 {published data only}
-
- Lacha J, Bartosova K, Lyerova L, Burgelova M, Teplan V, Vitko S. Long‐term effect of zenapax versus okt‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):265. [CENTRAL: CN‐00509303] - PubMed
-
- Lacha J, Simova M, Noskova L, Teplan V, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients. Transplantation Proceedings 2001;33(3):2273‐4. [MEDLINE: ] - PubMed
-
- Lacha J, Simova M, Noskova L, Teplan V, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. Transplantation 2000;69(8):S158. [CENTRAL: CN‐00401578] - PubMed
-
- Lacha J, Viklicky O, Noskova L, Kalanin J, Striz I, Vitko S. Zenapax versus OKT‐3 prophylaxis in immunologically high‐risk kidney transplant recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00401579]
Lawen 2003 {published data only}
-
- Davies E, Lawen J, Mourad G, Oppenheimer F, Durand D, Gonzalez‐Molina M, et al. Basiliximab (Simulect) is safe and effective in combination with neoral, steroids and cellcept for the prevention of acute rejection episodes in renal transplantation. Interim results of a double blind, randomized clinical trial [abstract]. American Society of Nephrology 1999;10(Program & Abstracts):725A‐6A. [CENTRAL: CN‐00400659]
-
- Lawen J, Davies E, Mourad G, Oppenheimer F, Gonzalez‐Molina M, Bourbigot B, et al. Basiliximab (Simulect) is safe and effective in combination with triple therapy of neoral steroids and cellcept in renal transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S260. [CENTRAL: CN‐00401599]
-
- Lawen JG, Davies EA, Mourad G, Oppenheimer F, Molina MG, Rostaing L, et al. Randomized double‐blind study of immunoprophylaxis with basiliximab, a chimeric anti‐interleukin‐2 receptor monoclonal antibody, in combination with mycophenolate mofetil‐containing triple therapy in renal transplantation. Transplantation 2003;75(1):37‐43. [MEDLINE: ] - PubMed
Lebranchu 2002 {published data only}
-
- Al Najjar A, Etienne I, Pogamp P, Bridoux F, Meur Y, Toupance O, et al. Long‐term results of monoclonal anti‐Il2‐receptor antibody versus polyclonal antilymphocyte antibodies as induction therapy in renal transplantation. Transplantation Proceedings 2006;38(7):2298‐9. [MEDLINE: ] - PubMed
-
- Brun C, Al Najjar A, Buchler M, Pen C, Lebranchu Y, Lilliu H. Cost‐minimisation study comparing simulect versus thymoglobuline in renal transplant induction. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00509107] - PubMed
-
- Buchler M, Benfatma L, Lepogamp P, Bridoux F, Lemeur Y, Toupance O, et al. Three year results of a randomized study comparing as induction treatment simulect® and thymoglobuline®. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):349. [CENTRAL: CN‐00509108]
-
- Lebranchu Y, Bridoux F, Buchler M, Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF‐containing triple therapy. American Journal of Transplantation 2002;2(1):48‐56. [MEDLINE: ] - PubMed
-
- Lebranchu Y, Bridoux F, Etienne I, Buchler M, Toupance O, Meur Y, et al. A multicenter, randomized trial of Simulect versus thymoglobuline in renal transplantation [abstract no:1598]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00401605]
Lin 2006 {published data only}
-
- Lin M, Ming A, Zhao M. The clinical study of two‐dose basiliximab compared with two‐dose daclizumab in renal transplantation [abstract]. Transplantation 2004;78(2):466. [CENTRAL: CN‐00509323] - PubMed
-
- Lin M, Ming A, Zhao M. Two‐dose basiliximab compared with two‐dose daclizumab in renal transplantation: a clinical study. Clinical Transplantation 2006;20(3):325‐9. [MEDLINE: ] - PubMed
Locke 2008 {published data only}
-
- Leffell MS, Kopchliiska D, Lucas DP, Jackson AM, Montgomery RA, Locke JE, et al. Effect of induction agent on cellular and humoral responses to renal transplants in sensitized patients [abstract no: 14]. American Journal of Transplantation 2008;8(Suppl 2):182.
-
- Locke J, Simpkins C, Leffell MS, Zacary A, Collins V, Warren D, et al. Results of a randomized prospective study of induction therapy with daclizumab versus thymoglobulin among crossmatch positive renal transplant recipients [abstract no: 521]. Transplantation 2008;86(Suppl 2):182‐3.
Martin Garcia 2003 {published data only}
-
- Martin GD, Martin GJ, Mendiluce A, Gordillo R, Bustamente J. Tacrolimus‐basiliximab versus cyclosporine‐basiliximab in renal transplantation "de novo": acute rejection and complications. Transplantation Proceedings 2003;35(5):1694‐6. [MEDLINE: ] - PubMed
Matl 2001 {published data only}
-
- Matl I, Bachleda P, Lao M, Michalsky R, Navratil P, Treska V. Basiliximab (Simulect) can be administered safely and effectively by IV bolus in a single dose on day 1 post renal transplantation in patients receiving triple therapy with azathioprine [abstract no:1107]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00401865] - PubMed
-
- Matl I, Bachleda P, Lao M, Michalsky R, Navratil P, Treska V, et al. Safety and efficacy of an alternative basiliximab (Simulect) regimen after renal transplantation: administration of a single 40‐mg dose on the first postoperative day in patients receiving triple therapy with azathioprine. Transplant International 2003;16(1):45‐52. [MEDLINE: ] - PubMed
-
- Matl I, Bachleda P, Michalsky R, Navratil P, Lao M, Treska V, et al. Basiliximab can be administered safely and effectively in a single dose on day 1 postrenal transplantation in patients receiving triple therapy with azathioprine. Transplantation Proceedings 2001;33(7‐8):3205‐6. [MEDLINE: ] - PubMed
Mourad 2004 {published data only}
-
- Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal‐transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78(4):584‐590. [MEDLINE: ] - PubMed
-
- Mourad G, Rostaing L, Legendre C, Lorho R, Therver E, Fares N. Simulect versus thymoglobulin with delayed introduction of neoral in renal transplantation: three month results of a French multicenter randomized trial [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00402018]
-
- Mourad GJ, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. A sequential protocol using simulect vs thymoglobulin in low immunological risk renal transplant recipients: six‐month results of a French multicenter, randomized trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00446849]
Nair 2001 {published data only}
-
- Nair MP, Nampoory MR, Johny KV, Costandi JN, Abdulhalim M, Reshaid W, et al. Induction immunosuppression with interleukin‐2 receptor antibodies (basiliximab and daclizumab) in renal transplant recipients. Transplantation Proceedings 2001;33(5):2767‐2769. - PubMed
-
- Nampoory MR, Abdulhalim M, Johny KV, Jawad Donia FA, Nair MP, Said T, et al. Bolus anti‐thymocyte globulin induction in renal transplant recipients: a comparison with conventional ATG or anti‐interleukin‐2 receptor antibody induction. Transplantation Proceedings 2002;34(7):2916‐9. [MEDLINE: ] - PubMed
-
- Nampoory NMR, Nair MP, Johny KV, Said T, El‐Reshaid W, Samhan M, et al. Induction immunosuppression with anti interleukin (IL‐2) receptor antibodies and anti thymocyte globulin in renal transplantation ‐ a comparative study [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):699A‐700A. [CENTRAL: CN‐00433639]
Nashan 1997 {published data only}
-
- Akehurst R, Chilcott J, Holmes M. The economic implications of the use of Basiliximab versus placebo for the control of acute cellular rejection in renal allograft recipients [abstract]. Transplantation 1999;67(7):S155. [CENTRAL: CN‐00400025]
-
- Breidenbach T, Korn A, Maibucher A, Schlitt HJ, Oldhafer KJ, Kliem V, et al. Two years results of a clinical trial with basiliximab (Simulect) in renal transplantation [abstract]. XVII World Congress of the Transplantation Society; 1998 Jul 12‐17; Montreal, Canada. 1998. [CENTRAL: CN‐00400373]
-
- Breidenbach T, Korn A, Schlitt HJ, Kliem V, Brunkhorst R, Schmidt AG, et al. Basiliximab (Simulect) reduces acute rejections, CMV infections and duration of hospital stay in renal allograft patients [abstract]. Transplantation 1998;65(12):S180. [CENTRAL: CN‐00400374]
-
- Chilcott J, Akehurst R, Whitfield M. Economics of Basiliximab (Simulect) in preventing acute rejection in renal transplantation [abstract no:1453]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400541]
-
- Chilcott JB, Holmes MW, Walters S, Akehurst RL, Nashan B. The economics of basiliximab (Simulect) in preventing acute rejection in renal transplantation. Transplant International 2002;15(9‐10):486‐93. [MEDLINE: ] - PubMed
Noel 2009 {published data only}
-
- Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus thymoglobulin in renal transplant recipients with a high immunological risk: a French and Belgian prospective randomized trial [abstract no: 331]. American Journal of Transplantation 2007;7(Suppl 2):233. [CENTRAL: CN‐00644178]
Offner 2008 {published data only}
-
- Hocker B, Kovarik J, Offner GF, Zimmerhack LB, Jungraithmayr TC, Koepf S, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients under CsA, MMF and corticosteroids [abstract no: COD. PP 210]. Pediatric Nephrology 2006;21(10):1574.
-
- Hocker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation 2008;86(9):1234‐40. [MEDLINE: ] - PubMed
-
- Offner G, Toenshoff B, Hocker B, Krauss M, Bulla M, Cochat P, et al. Efficacy and safety of basiliximab in pediatric renal transplant patients receiving cyclosporine, mycophenolate mofetil, and steroids. Transplantation 2008;86(9):1241‐8. [MEDLINE: ] - PubMed
-
- Tönshoff B, Offner G, Hoecker B, Pape L, Rascher W, Neuhaus T, et al. A multicenter, placebo‐controlled trial evaluating the efficacy and safety of Basiliximab (Simulect) in combination with CsA, MMF and steroids in pediatric renal allograft recipients: 12 months results [abstract no: COD. OP 25]. Pediatric Nephrology 2006;21(10):1513. [CENTRAL: CN‐00583475]
-
- Zimmerhackl LB, Grossmann A, Jungraithmayr TC, Pedevilla P, Cochat P, Doetsch J, et al. Basiliximab as induction therapy in pediatric renal transplantation: 5 year results [abstract no: SA‐PO2534]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):679A.
Parrott 2005 {published data only}
-
- Parrott NR, Hammad AQ, Watson CJ, Lodge JP, Andrews CD. Multicenter, randomized study of the effectiveness of basiliximab in avoiding addition of steroids to cyclosporine a monotherapy in renal transplant recipients. Transplantation 2005;79(3):344‐348. [MEDLINE: ] - PubMed
-
- Parrott NR, Hammad AQ, Watson CJE, Lodge PJA, Andrews C. Basiliximab (simulect) with ciclosporin (neoral) as a strategy for steroid avoidance in renal transplantation. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):350. [CENTRAL: CN‐00509403]
Perrea 2006 {published data only}
-
- Perrea DN, Moulakakis KG, Poulakou MV, Vlachos IS, Papachristodoulou A, Kostakis AI. Correlation between oxidative stress and immunosuppressive therapy in renal transplant recipients with an uneventful postoperative course and stable renal function. International Urology & Nephrology 2006;38(2):343‐8. [MEDLINE: ] - PubMed
Pescovitz 2003 {published data only}
-
- Kirkman RL, Vincenti F, Pescovitz MD, Bumgardner GL, Gaston RS, Light SE. A phase I/II randomized, double blind, placebo controlled study of zenapax in combination with cellcept, neoral, and steroids. [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:260.
-
- Pescovitz MD, Bumgardner G, Gaston RS, Kirkman RL, Light S, Patel IH, et al. Pharmacokinetics of daclizumab and mycophenolate mofetil with cyclosporine and steroids in renal transplantation. Clinical Transplantation 2003;17(6):511‐7. [MEDLINE: ] - PubMed
Philosophe 2002 {published data only}
-
- Philosophe B, Schweitzer EJ, Foster CE, Campos L, Myers S, Bartlett ST. Long term results of a prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no: 126]. American Journal of Transplantation 2005;5(Suppl 11):188. [CENTRAL: CN‐00644144]
-
- Philosophe B, Wiland AM, Mann DL, Farney AC, Schweitzer EJ, Colonna JO, et al. Prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no:2063]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
-
- Philosophe B, Wiland AM, Mann DL, Farney AC, Schweitzer EJ, Colonna JO, et al. Prospective randomized study comparing OKT3 and a truncated daclizumab regimen as induction for marginal kidneys at high risk for delayed graft function [abstract no:402]. American Journal of Transplantation 2002;2(Suppl 3):239. [CENTRAL: CN‐00402238]
Pisani 2001 {published data only}
-
- Coppelli A, Buonomo O, Iaria G, Pisani F, Pollicita S, Rizzello A. Preliminary results of a prospective randomized study of basiliximab and steroid withdrawal in kidney transplantation [abstract no:1617]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400600]
-
- Pisani F, Buonomo O, Iaria G, Tisone G, Mazzarella V, Pollicita S, et al. Preliminary results of a prospective randomized study of basiliximab in kidney transplantation. Transplantation Proceedings 2001;33(1‐2):2032‐3. [MEDLINE: ] - PubMed
Ponticelli 2001 {published data only}
-
- Chilcott, JB. Economics of basiliximab (Simulect) in preventing acute rejection in renal transplantation. ISOT 2001. - PubMed
-
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] - PubMed
-
- Ponticelli C, Cambi V, Shapira Z, Monteon F, Salmela K, Kahn D, et al. A multicenter, double blind, placebo controlled study of basiliximab (simulect) in combination with triple therapy including azathioprine for the prevention of acute rejection episodes in renal allograft patients [abstract]. Transplantation 1999;67(7):S158. [CENTRAL: CN‐00402269]
-
- Ponticelli C, Yusim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab (Simulect) significantly reduces the incidence of acute rejection in renal transplant patients receiving triple therapy with azathioprine [abstract]. Transplantation 2000;69(8 Suppl):S156. [CENTRAL: CN‐00402270] - PubMed
-
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab (Simulect) significantly reduces the incidence of acute rejection in renal transplant patients receiving a triple therapy with azathioprine [abstract]. International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000.
Pourfarziani 2003 {published data only}
-
- Pourfarziani V, Lesanpezeshki M, Einollahi B, et al. Zenapax versus ALG prophylaxis in immunologically high‐risk group of renal allograft recipients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):494. [CENTRAL: CN‐00447271]
-
- Pourfarziani V, Lesanpezeshki M, Eyn EB. Zenapax versus anti‐lymphocyte globulin prophylaxis in immunologically high‐risk group of renal allograft recipients. Kowsar Medical Journal 2007;12(1):69‐73.
Ruggenenti 2006 {published data only}
-
- Codreanu I, Cravedi P, Ruggenenti P, Remuzzi G. Antilymphocyte therapy in kidney transplantation: a prospective randomized trial of full‐dose rabbit anti‐human thymocyte globulin (ratg) versus low‐dose RATG and basiliximab. [abstract]. Transplantation 2004;78(2 Suppl):276. [CENTRAL: CN‐00509138]
-
- Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G. Basiliximab combined with low‐dose rabbit anti‐human thymocyte globulin: A possible further step toward effective and minimally toxic T cell‐targeted therapy in kidney transplantation. Clinical Journal of the American Society of Nephrology ‐ CJASN 2006;1(3):546‐54. [MEDLINE: ] - PubMed
Sandrini 2002 {published data only}
-
- Boggi U, Arisi L, Valente U, Greca G, Calconi G, Donati D, et al. Basiliximab facilitates steroid withdrawal after primary kidney transplantation: results of a placebo‐controlled study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00400327]
-
- Sandrini S, Arisi L, Rizzo G, Valente U, Greca G, Calconi G, et al. Simulect facilitates steroid withdrawal after renal transplantation: results of an Italian, multicentre, placebo‐controlled study [abstract]. 5th International Conference on New Trends in Clinical and Experimental Immunosuppression; 2002 Feb 7‐10; Geneva, Switzerland. 2002. [CENTRAL: CN‐00402503]
-
- Sandrini S, Rizzo G, Valente U, Greca G, Calconi G, Donati D, et al. Basiliximab facilitates steroid withdrawal after renal transplantation: results of an Italian, multicentre, placebo‐controlled study (Swiss study) [abstract]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00403504]
Sheashaa 2003 {published data only}
-
- Sheashaa HA, Bakr MA, Ismail AM, Gheith OE, Dahshan KF, Sobh MA, et al. Long‐term evaluation of basiliximab induction therapy in live donor kidney transplantation: a five‐year prospective randomized study. American Journal of Nephrology 2005;25(3):221‐5. [MEDLINE: ] - PubMed
-
- Sheashaa HA, Bakr MA, Ismail AM, Gheith OE, El‐Dahshan KF, Sobh MA, et al. Basiliximab reduces the incidence of acute cellular rejection in live related donor kidney transplantation, results of five years prospective randomized trial [abstract no:SP425]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v161. [CENTRAL: CN‐00644283]
-
- Sheashaa HA, Bakr MA, Ismail AM, Mahmoud KM, Sobh MA, Ghoneim MA. Basiliximab induction therapy for live donor kidney transplantation: a long‐term follow‐up of prospective randomized controlled study. Clinical & Experimental Nephrology 2008;12(5):376‐81. [MEDLINE: ] - PubMed
-
- Sheashaa HA, Bakr MA, Ismail AM, Sobh MA, Ghoneim MA. Basiliximab reduces the incidence of acute cellular rejection in live‐related‐donor kidney transplantation: a three‐year prospective randomized trial. Journal of Nephrology 2003 May;16(3):393‐8. [MEDLINE: ] - PubMed
Shidban 2000 {published data only}
-
- Shidban H, Sabawi M, Aswad S, Chambers G, Castillon I, Naraghi R, et al. Controlled trial of IL2R antibody basiliximab (Simulect) vs low dose OKT3 in cadaver kidney transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S156. [CENTRAL: CN‐00402633]
Shidban 2003 {published data only}
-
- Aswad S, Shidban H, Naraghi R, Puhawan M, Sabawi M, Mendez RG, et al. A prospective, randomized, phase IV comparative trial of Thymoglobulin® versus Simulect® for the prevention of delayed graft function and acute allograft rejection in renal transplant recipients. [abstract no: SA‐PO551]. Journal of the American Society of Nephrology 2003;14(Nov):417A. [CENTRAL: CN‐00447713]
-
- Shidban H, Sabawi M, Puhawan M, Aswad S, Mendez RG, Mendez R. A prospective, randomized, phase IV comparative trial of thymoglobulin versus simulect for the prevention of delayed graft function and acute allograft rejection in renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):352. [CENTRAL: CN‐00447713]
Sollinger 2001 {published data only}
-
- Kaplan B, Polsky D, Weinfurt K, Fastenau J, Kim J, Ryu S, et al. Quality of life improvement and lower costs associated with Simulect based induction therapy [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):733A. [CENTRAL: CN‐00401459]
-
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] - PubMed
-
- Pescovitz M, Kovarik JM, Gerbeau C, Simulect US‐O1 Study Group. Pharmacokinetics of basiliximab when coadministered with MMF in kidney transplantation [abstract no: 0112]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00402225]
-
- Pescovitz MD, Barbeito R. Effect of "C2" Cyclosporine Levels and Time to Initiation of Cyclosporine Therapy on Outcomes in Patients Receiving Neoral and Simulect [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):703A. [CENTRAL: CN‐00433641]
-
- Pescovitz MD, Barbeito R, Simulect US. Two‐hour post‐dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clinical Transplantation 2002;16(5):378‐82. [MEDLINE: ] - PubMed
Soulillou/Cant 1990 {published data only}
-
- Cantarovich D, Giral M, Hourmant M, Dantal J, Blancho G, Soulillou JP. 15‐year results of a randomized study comparing anti‐CD25 monoclonal antibody and antithymocyte globulin induction in kidney transplantation [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002.
-
- Cantarovich D, Mauff B, Hourmant M, Giral M, Denis M, Jacques Y, et al. Anti‐IL2 receptor monoclonal antibody (33B3.1) in prophylaxis of early kidney rejection in humans: a randomized trial versus rabbit antithymocyte globulin. Transplantation Proceedings 1989;21(1 (Pt 2)):1769‐71. [MEDLINE: ] - PubMed
-
- Cantarovich M, Giral M, Hourmant M, Dantal J, Blancho G, Soulillou JP. 15‐year results of a randomized study comparing anti‐cd25 monoclonal antibody and antithymocyte globulin induction in kidney transplantation. [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):308‐9. [CENTRAL: CN‐00415383]
-
- Soulillou JP, Cantarovich D, Mauff B, Giral M, Robillard N, Hourmant M, et al. Randomized controlled trial of a monoclonal antibody against the interleukin‐2 receptor (33B3.1) as compared with rabbit antithymocyte globulin for prophylaxis against rejection of renal allografts. New England Journal of Medicine 1990;322(17):1175‐82. [MEDLINE: ] - PubMed
SYMPHONY (Ekberg) 2007 {published data only}
-
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981]
-
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐00602015]
-
- Ekberg H, Bernasconi C, Halloran P. CNI minimisation with 2 G mycophenolate mofetil ‐ what have we learned from the Symphony Study [abstract no: 964]. Transplantation 2008;86(2 Suppl):334. [CENTRAL: CN‐00671785]
-
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retained their distinct toxicity profiles despite low doses in the Symphony Study [abstract no: 55]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653721] - PubMed
-
- Ekberg H, Mamelok R, Bernasconi C, Vincenti F, Tedesco‐Silva H, Daloze P, et al. The challenge of meeting target drug concentrations: experience from the Symphony study [abstract no:58]. American Journal of Transplantation 2007;7(Suppl 2):161. [CENTRAL: CN‐00615834]
Tan 2004 {published data only}
-
- Tan J, Yang S, Wu W. Basiliximab (Simulect) reduces acute rejection among sensitized kidney allograft recipients. Transplantation Proceedings 2005;37(2):903‐5. [MEDLINE: ] - PubMed
-
- Tan J, Yang S, Wu W. Basiliximab (simulect®) reduces acute rejection among sensitized kidney allograft recipients [abstract]. Transplantation 2004;78(2 Suppl):266. - PubMed
ter Meulen 2002 {published data only}
-
- Hendrikx TK, Klepper M, IJzermans J, Weimar W, Baan CC. Clinical rejection and persistent immune regulation in kidney transplant patients. Transplant Immunology 2009;21(3):129‐35. [MEDLINE: ] - PubMed
-
- Hesselink DA, Ngyuen H, Wabbijn M, Smak Gregoor PJH, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids [abstract]. American Journal of Transplantation 2003;3(Suppl 5):482. - PMC - PubMed
-
- Ter Meulen CG, Riemsdijk IC, Hene RJ, Christiaans MHL, Gelder T, Hilbrands LB, et al. A prospective randomized trial comparing steroid‐free immunosuppression with limited steroid exposure on bone mineral density in the first year after renal transplantation [abstract no:0344]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00402832]
-
- ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin‐2 receptor alpha after treatment with daclizumab. Kidney International 2003;64(2):697‐703. [MEDLINE: ] - PubMed
Tullius 2003 {published data only}
-
- Pascher A, Ulrich F, Kohler S, Weiss S, Tullius S, Reinke P, et al. ATG versus basiliximab induction therapy in kidney allograft recipients receiving dual immunosuppressive regimen: six‐year results [abstract no: 800]. Transplantation 2008;86(2 Suppl):279. [CENTRAL: CN‐00676048]
-
- Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. ATG versus basiliximab induction therapy in renal allograft recipients receiving a dual immunosuppressive regimen: one‐year results. Transplantation Proceedings 2003;35(6):2100‐1. [MEDLINE: ] - PubMed
-
- Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. Induction therapy with ATG vs basiliximab (simulect) in renal allograft recipients: 1‐year results of a prospective randomized, single center study [abstract]. American Journal of Transplantation 2003;3(Suppl 5):478. [CENTRAL: CN‐00520398]
-
- Ulrich F, Niedzwiecki S, Pascher A, Fellmer P, Weiss S, Kohler S, et al. ATG versus basiliximab induction therapy in kidney allograft recipients receiving a dual immunosuppressive regimen: six‐year results [abstract no: 540]. American Journal of Transplantation 2008;8(Suppl 2):322. [CENTRAL: CN‐00653718]
van Gelder 1995 {published data only}
-
- Wabbijn M, Balk AH, Domburg RT, Vantrimpont PJ, Riemsdijk IC, Baan CC, et al. Ten‐year follow‐up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin‐2 receptor. Experimental & Clinical Transplantation 2004;2(1):201‐7. [MEDLINE: ] - PubMed
-
- Gelder T, Zietse R, Mulder AH, Yzermans JN, Hesse CJ, Vaessen LM, et al. A double‐blind, placebo‐controlled study of monoclonal anti‐interleukin‐2 receptor antibody (BT563) administration to prevent acute rejection after kidney transplantation. Transplantation 1995;60(3):248‐52. [MEDLINE: ] - PubMed
-
- Gelder T, Zietse R, Yzermans JN, Rischen‐Vos J, Vaessen LM, Weimar W. Long‐term follow‐up after induction treatment with monoclonal anti‐interleukin‐2 receptor antibody (BT563) in kidney allograft recipients: a double‐blind, placebo‐controlled trial. Transplantation Proceedings 1996;28(6):3221‐2. [MEDLINE: ] - PubMed
Vincenti 2003 {published data only}
-
- Vincenti F, Pace D, Birnbaum J, Lantz M. Pharmacokinetic and pharmacodynamic studies of one or two doses of daclizumab in renal transplantation. American Journal of Transplantation 2003;3(1):50‐2. [MEDLINE: ] - PubMed
Wilson 2004 {published data only}
-
- Asher JF, Wilson CH, Gupta A, Gok MA, Talbot D. Use of daclizumab in preventing delayed graft function in non‐heart beating donor kidney transplantation in Newcastle upon Tyne. Transplantationsmedizin ‐ Organ der Deutschen Transplantationsgesellschaft 2004;16(2):96‐100. [EMBASE: 2004360749]
-
- Asir L, Wilson CH, Talbot D. Interleukin 2 receptor blockers may directly inhibit lymphocyte mediated ischaemia reperfusion injury. Transplant International 2005 Sep;18(9):1116. [MEDLINE: ] - PubMed
-
- Wilson C, Brook NR, Gok MA, Gupta A, Asher JF, Nicholson ML, et al. Evaluation of daclizumab to reduce delayed graft function in non‐heart‐beating renal transplantation: a prospective, randomized trial. Transplantation Proceedings 2005;37(4):1774‐5. [MEDLINE: ] - PubMed
-
- Wilson C, Brook NR, Gok MA, Gupta AJ, Asher J, Nicholson ML, et al. Evaluation of daclizumab to reduce delayed graft function in non‐heartbeating renal transplantation: a prospective randomised trial [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550743]
-
- Wilson CH, Brook NR, Gok MA, Asher JF, Nicholson ML, Talbot D. Randomized clinical trial of daclizumab induction and delayed introduction of tacrolimus for recipients of non‐heart‐beating kidney transplants. British Journal of Surgery 2005;92(6):681‐7. [MEDLINE: ] - PubMed
Yussim 2004 {published data only}
-
- Yussim A, Bielsky V, Bar‐Nathan N, Shaharabani E, Burstein I, Lustig S, et al. Two‐dose daclizumab in conjunction with tacrolimus‐based protocol in kidney transplantation ‐ prospective, randomized study. [abstract]. Transplantation 2004;78(2 Suppl):466. [CENTRAL: CN‐00509575]
References to studies excluded from this review
Andres 2009 {published data only}
-
- Andres A, Marcen R, Valdes F, Plumed JS, Sola R, Errasti P, et al. A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function. Clinical Transplantation 2009;23(1):23‐32. [MEDLINE: ] - PubMed
Budde 2005 {published data only}
-
- Budde K, Bosmans J, Zeier M, Sennesael J, Hopt U, Fischer WH, et al. Safety and efficacy of reduced or full dose of cyclosporine (neoral®) in combination with mycophenolatesodium (myfortic®), basiliximab (simulect®), and steroids in de novo kidney transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):83. [CENTRAL: CN‐00527096]
-
- Budde K, Bosmans JL, Sennesael J, Zeier M, Hopt U, Fischer W, et al. Reduced cyclosporine exposure is safe and efficacious in combination with basiliximab, enteric‐coated mycophenolate‐sodium, and steroids [abstract no: 1195]. American Journal of Transplantation 2005;5(Suppl 11):461. [CENTRAL: CN‐00644165]
-
- Budde K, Bosmans JL, Sennesael J, Zeier M, Pisarski P, Schutz M, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab. Clinical Nephrology 2007;67(3):164‐75. [MEDLINE: ] - PubMed
-
- Budde K, Zeier M, Bosmans JL, Sennesael J, Glander P, Fischer W, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab [abstract no: F‐PO1088]. Journal of the American Society of Nephrology 2006;17(Abstracts):565A. [CENTRAL: CN‐00644166] - PubMed
Burke 2005 {published data only}
-
- Burke GW III, Ciancio G, Figueiro J, Olson L, Gomez C, Rosen A, et al. Can acute rejection be prevented in SPK transplantation?. Transplantation Proceedings 2002;34(5):1913‐4. [MEDLINE: ] - PubMed
-
- Burke GW, Ciancio G, Mattaiazzi A, Gomez C, Rosen A, Suzart K, et al. Can acute rejection be prevented in SPK transplantation? A randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance, comparing rapamycin with mycophenolate mofetil [abstract]. American Journal of Transplantation 2003;3(Suppl 5):322. [CENTRAL: CN‐00444587]
-
- Burke GW, Ciancio G, Mattiazzi A, Gomez C, Rosen A, Miller J. Can acute rejection be prevented in SPK transplantation? a randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycophenolate mofetil. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):562. [CENTRAL: CN‐00509113]
-
- Burke GW, Ciancio G, Mattiazzi A, Gomez C, Rosen A, Miller J. Lower rate of acute rejection with rapamycin than with mycophenolate mofetil in kidney pancreas transplantation: a randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycopenolate mofetil [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550658]
-
- Burke GW, Ciancio G, Mattiazzi A, Illanes H, Gomez C, Rosen A, et al. Lower rate of acute rejection with rapamycin than with mycophenolate mofetil in kidney pancreas transplantation. A randomized, prospective study with thymoglobulin/zenapax induction, tacrolimus and steroid maintenance: comparing rapamycin with mycophenolate mofetil. [abstract no: 789]. American Journal of Transplantation 2005;5(Suppl 11):357. [CENTRAL: CN‐00644167]
Chadban 2006 {published data only}
-
- Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one‐year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric‐coated mycophenolate sodium (EC‐MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA‐ME), basiliximab and steroids in de novo kidney transplantation. [abstract no: 32]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:51. [CENTRAL: CN‐00583470]
Chan 2008 {published data only}
-
- Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85(6):821‐6. [MEDLINE: ] - PubMed
-
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Everolimus (RAD001) concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients [abstract no: SA‐PO2529]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):678A.
Flechner‐318 2002 {published data only}
-
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351]
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00415657]
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches. [abstract no:1317]. American Journal of Transplantation 2002;2(Suppl 3):470.
-
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] - PubMed
-
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil‐based immunosuppression: 5‐year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83(7):883‐92. [MEDLINE: ] - PubMed
FREEDOM Study {published data only}
-
- Schena FP, Vincenti F, Paraskevas S, Hauser I, FREEDOM Study Group. Renal function and rejection incidence in de novo renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids [abstract no: F‐FC153]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601969]
-
- Schena FP, Vincenti F, Paraskevas S, Hauser I, Grinyo J, FREEDOM Study Group. 12‐month results of a prospective, randomized trial of steroid avoidance, steroid withdrawal and standard steroids in de novo renal transplant patients receiving cyclosporine, enteric‐coated mycophenolate sodium (EC‐MPS, myfortic®) and basiliximab [abstract no: 54]. American Journal of Transplantation 2006;6(Suppl 2):84‐5. [CENTRAL: CN‐00644263]
-
- Vincenti F, Schena FP, Paraskevas S, Hauser I, FREEDOM Study Group. Comparison of metabolic parameters in renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids with a 12‐month, randomized, multicenter trial [abstract no: F‐PO1076]. Journal of the American Society of Nephrology 2006;17(Abstracts):562A. [CENTRAL: CN‐00602097]
-
- Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. American Journal of Transplantation 2008;8(2):307‐16. [MEDLINE: ] - PubMed
-
- Walker R, Campbell S, Chadban S, Kanellis J, Pilmore H, Russ G. Preliminary results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS), basiliximab, and neoral C‐2 comparing a regimen without steroids or short‐term use of steroids with standard steroid treatment in de novo kidney recipients. [abstract no: 34]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:52. [CENTRAL: CN‐00583481]
Hamdy 2005 {published data only}
-
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy e, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] - PubMed
Hiesse 1992 {published data only}
-
- Hiesse C, Kriaa F, Alard P, Lantz O, Noury J, Bensadoun H, et al. Prophylactic use of the IL‐2 receptor‐specific monoclonal antibody LO‐Tact‐1 with cyclosporin A and steroids in renal transplantation. Transplant International 1992;5 Suppl 1:S444‐7. [MEDLINE: ] - PubMed
Hirose 2004 {published data only}
-
- Hirose K, Posselt AM, Stock PG, Hirose R, Vincenti F. Treatment of kidney transplant patients with the novel co‐stimulatory blocker LEA29y (BMS‐224818) and antiil2 receptor antibody does not impede the development of regulatory t cells. [abstract]. American Journal of Transplantation 2004;4(Suppl 8):442. [CENTRAL: CN‐00509233]
Kovarik 2003 {published data only}
-
- Kovarik JM, Dantal J, Civati G, Rizzo G, Rouilly M, Bettoni‐Ristic O, et al. Influence of delayed initiation of cyclosporine on everolimus pharmacokinetics in de novo renal transplant patients. American Journal of Transplantation 2003;3(12):1576‐80. [MEDLINE: ] - PubMed
Kramer‐2307 2003 {published data only}
-
- Campbell S, Eris J, Brown F, Russ G, Caicedo L, Walker R, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 24 month result [abstract no: FC‐50002]. Nephrology 2005;10(Suppl):A1. [CENTRAL: CN‐00602128]
-
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CsA and basiliximab at 24 months. [abstract no: 36]. 24th Annual Scientific Meeting.Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:53. [CENTRAL: CN‐00583469]
-
- Eris J, Campbell S, Burbigott B, Leone J, Kraemer B, Rigotti P, et al. Excellent graft function in de novo kidney transplant recipients treated with certican®, simulect® and reduced neoral® exposure: 12‐month results [abstract]. Transplantation 2004;78(2 Suppl):31.
-
- Eris J, Campbell S, Walker R, Russ G, Stambe C, RAD2307 International Study Group. Excellent graft function in de novo transplant recipients treated with everolimus, reduced dose neoral and simulect: 6 months analysis. [abstract]. 22nd Annual Scientific Meeting Transplantation Society of Australia & New Zealand; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:33. [CENTRAL: CN‐00509178]
-
- Kraemer BK, Bourbigot B, Vitko S, Rigotti P, Caicedo L, Boccardo G. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CSA and basiliximab at 24 months [abstract no: PO‐437]. 12th Congress of the European Society for Organ Transplantation (ESOT); 2005 Oct 15‐19; Geneva, Switzerland. 2005. [CENTRAL: CN‐00653704]
Kreis 2003 {published data only}
-
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Lowering cyclosporine dose is not associated with an increased risk of gastro‐intestinal adverse events nor the need for dosage decrease of mycophenolate mofetil [abstract no: P743]. Transplantation 2004;78(2 Suppl):462. [CENTRAL: CN‐00509292]
-
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse m, et al. Daclizumab and mycophenolate mofetil in renal transplant recipients: 2‐year outcome after early reduction of cyclosporine [abstract]. American Journal of Transplantation 2003;3(Suppl 5):476. [CENTRAL: CN‐00446199]
Light 2002 {published data only}
-
- Light JA, Sasaki TM, Ghasemian R, Barhyte DY, Fowlkes DL. Daclizumab induction/tacrolimus sparing: a randomized prospective trial in renal transplantation. Clinical Transplantation 2002;16(Suppl 7):30‐3. [MEDLINE: ] - PubMed
Martinez‐Mier 2006 {published data only}
-
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: Initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [EMBASE: 2006628001] - PubMed
McDonald 2008 {published data only}
-
- McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. American Journal of Transplantation 2008;8(5):984‐9. [MEDLINE: ] - PubMed
Meier‐Kriesche 2004 {published data only}
-
- Bresnahan B, Cibrik D, Jensik S, Whelchel J, Klintmalm G, Cohen D, et al. Treatment of high‐risk renal transplant recipients with EC‐MPS (Myfortic®) is safe and efficacious [abstract no: PUB216]. Journal of the American Society of Nephrology 2005;16:829A. [CENTRAL: CN‐00644277]
-
- Cibrik D, Jensik S, Bresnahan B, Whelchel J, Klintmalm G, ERL2405‐US01 Study Group. Safety and efficacy of EC‐MPS in combination with simulect and neoral in de novo renal transplant high‐risk recipients [abstract no: 135]. American Journal of Transplantation 2005;5(Suppl 11):190. [CENTRAL: CN‐00644170]
-
- Cibrik D, Jensik S, Meier‐Kriesche H, Bresnahan B, Lieberman B, Myfortic US01 renal Transplant Group. Enteric‐coated mycophenolate sodium in combination with optimized neoral dosing, basiliximab, and steroids results in good efficacy and renal function in renal transplant recipients in the first six months [abstract no:220]. American Journal of Transplantation 2008;4(Suppl 8):218. [CENTRAL: CN‐00644278]
-
- Cibrik D, Meier‐Kriesche HU, Bresnahan B, Wu YM, Klintmalm G, Kew CE, et al. Renal function with cyclosporine C2 monitoring, enteric‐coated mycophenolate sodium and basiliximab: a 12‐month randomized trial in renal transplant recipients. Clinical Transplantation 2007;21(2):192‐201. [MEDLINE: ] - PubMed
-
- Meier‐Kriesche H, Cibrik D, Bresnahan B, Cohen D, Lieberman B. Optimized Neoral C2 monitoring in combination with enteric‐coated mycophenolic acid, basiliximab and steroids is effective, safe and tolerable: 12‐month results of a multicenter, randomized, prospective trial. [abstract no: F‐PO1068]. Journal of the American Society of Nephrology 2004;15(Oct Abstracts Issue):299A. [CENTRAL: CN‐00583408]
Montagnino 2005 {published data only}
-
- Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplantation Proceedings 2005;37(2):788‐90. [MEDLINE: ] - PubMed
-
- Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, et al. A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low‐dose cyclosporine. Nephrology Dialysis Transplantation 2008;23(2):707‐14. [MEDLINE: ] - PubMed
Mourad 2005 {published data only}
-
- Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. American Journal of Transplantation 2006;6(5 Pt 1):1042‐8. [MEDLINE: ] - PubMed
-
- Mourad G, Karras A, Kamar N, Garrigue V, Legendre C, Lefrancois N, et al. Renal function with delayed or immediate cyclosporine microemulsion in combination with enteric‐coated mycophenolate sodium and steroids: results of follow up to 30 months post‐transplant. Clinical Transplantation 2007;21(3):295‐300. [MEDLINE: ] - PubMed
-
- Mourad G, Rostaing L, Legendre C. Assessment of two strategies of neoral® administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic®, steroids and anti‐il2r antibodies: 6 months interim results. [abstract]. Transplantation 2004;78(2 Suppl):454. [CENTRAL: CN‐00509366] - PubMed
-
- Mourad G, Rostaing L, Legendre C, Myriade FR. Assessment of two strategies of neoral administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic, steroids, and anti‐IL2R antibodies: 12‐month results of a randomized, multicentre, open, prospective controlled study. Transplantation Proceedings 2005;37(2):920‐2. [MEDLINE: ] - PubMed
-
- Mourad G, Rostaing, Rostaing L, Legendre C. Assessment of two neoral® administration strategies on renal function and efficacy in de novo renal transplant patients receiving enteric‐coated mycophenolate sodium, steroids and anti‐il2r antibodies: 6 months interim analysis of a randomized, multicentre, open, prospective controlled study.[abstract]. American Journal of Transplantation 2004;4(Suppl 8):219. [CENTRAL: CN‐00509367]
MyPROMS Study {published data only}
-
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] - PubMed
Nematalla 2007 {published data only}
-
- Neamatalla A, Bakr A, Elagroudy A, Elshehawy E, Shokier A, Ghoneim M. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: FP222]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi93. [CENTRAL: CN‐00653762] - PubMed
-
- Nematalla AH, Bakr MA, Elagrody AE, Elshehawy E, Salim M, Shokier AA. Steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (one year follow up) [abstract no: SP734]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00653763] - PubMed
-
- Nematalla AH, Bakr MA, Gheith OA, Elagroudy AE, Elshahawy e, Aghoneim M. Steroid‐avoidance immunosuppression regimen in live‐donor renal allotransplant recipients: a prospective, randomized, controlled study. Experimental & Clinical Transplantation 2007;5(2):673‐9. [MEDLINE: ] - PubMed
Painter 2003 {published data only}
-
- Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, et al. Health‐related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney International 2003;63(6):2309‐16. [MEDLINE: ] - PubMed
Pescovitz 2004 {published data only}
-
- Pescovitz M, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety and efficacy of mycophenolate mofetil in combination with sirolimus vs cyclosporine in renal transplant patients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):251. [CENTRAL: CN‐00509411] - PMC - PubMed
-
- Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. British Journal of Clinical Pharmacology 2007;64(6):758‐71. [MEDLINE: ] - PMC - PubMed
Provenzano 2000 {published data only}
-
- Provenzano R, Tayeb J, Thackkar R, Morrison L. Analysis of patient and graft outcomes in daclizumab based induction immunosuppression using neoral vs tacrolimus [abstract]. Journal of the American Society of Nephrology 2000;11(Sept Program & Abstracts):703A. [CENTRAL: CN‐00433643]
Scholten 2006 {published data only}
-
- Scholten EM, Rowshani AT, Cremers S, Bemelman FJ, Eikmans M, Kan E, et al. Untreated rejection in 6‐month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of graft function. Journal of the American Society of Nephrology 2006;17(9):2622‐32. [MEDLINE: ] - PubMed
Tian 2007 {published data only}
-
- Tian J, Zhang JY, Hu D, Hu WF, Huang J. Influences of single‐dose Basiliximab on Foxp3mRNA, CD4+CD25+ regulatory T cells, interleukin‐2 and interleukin‐2 receptor in the peripheral blood of renal transplantation recipients. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(25):4861‐5. [EMBASE: 2007338028]
Vincenti 2005b {published data only}
-
- Vincenti F, Schena F, Walker R, Pescovitz M, Shoker A. 3 months interim results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS, Myfortic©), basiliximab, and neoral C‐2 comparing different steroid protocols in de novo kidney recipients [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2005;16(Oct):236A.
-
- Vincenti F, Schena FP, Walker R, Pescovitz MD, Shoker A, Grinyo J, et al. Preliminary 3‐month results comparing immunosuppressive regimens of enteric‐coated mycophenolate sodium (EC‐MPS) without steroids vs short‐term use of steroids vs standard steroid treatment including basiliximab, and neoral C‐2 in de novo kidney recipients [abstract no: 1542]. American Journal of Transplantation 2005;5(Suppl 11):548.
Wang 2008 {published data only}
-
- Wang Z, Xiao L, Shi BY, Qian YY, Bai HW, Chang JY, et al. Short‐term anti‐CD25 monoclonal antibody treatment and neogenetic CD4(+)CD25(high) regulatory T cells in kidney transplantation. Transplant Immunology 2008;19(1):69‐73. [MEDLINE: ] - PubMed
Zarkhin 2008 {published data only}
-
- Sarwal M, Zarkhin V, Mohile S, Kambham N, Li L, Martin J, et al. Randomized trial of Rituximab vs standard of care for B cell dense acute renal transplant rejection [abstract no: 538]. American Journal of Transplantation 2007;7(Suppl 2):287. [CENTRAL: CN‐00644179]
-
- Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. American Journal of Transplantation 2008;8(12):2607‐17. [MEDLINE: ] - PubMed
Additional references
ANZDATA 2008
-
- Campbell S, McDonald S, Excell L, Livingston B. Chapter 8 Transplantation. In: McDonald S, Excell L, Livingston B editor(s). ANZDATA Registry Report 2008. available from http://www.anzdata.org.au/v1/annual_reports_download.html. Adelaide, South Australia.: Australia and New Zealand Dialysis and Transplant Registry, 2008.
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [MEDLINE: ] - PubMed
Cibrik 2001
-
- Cibrik Dm, Kaplan B, Meier‐Kriesche H. Role of anti‐interleukin‐2 receptor antibodies in kidney transplantation. Biodrugs 2001;15(10):655‐66. [MEDLINE: ] - PubMed
Clarke 2001
-
- Clarke MJ. Obtaining individual patient data from randomised controlled trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care. BMJ books, 2001:109‐21.
Cuervo 2003
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Publishing Group, 2001:285‐312.
Dickersin 1994
Egger 1997
Glanville 2006
Goebel 2000
-
- Goebel J, Stevens E, Forrest K, Roszman TL. Daclizumab (Zenapax) inhibits early interleukin‐2 receptor signal transduction events. Transplant Immunology 2000;8(3):153‐9. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2008
-
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons Inc, 2008.
Hong 2000
-
- Hong J, Kahan B. Immunosuppressive agents in organ transplantation: past, present and future. Seminars in Nephrology 2000;20(2):108‐25. [MEDLINE: ] - PubMed
Lefebvre 1996
-
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium, Adelaide, Australia, 20‐24 October. 1996.
Lefebvre 2008
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [MEDLINE: ] - PubMed
Morton 2009
-
- Morton RL, Howard K, Webster AC, Wong G, Craig JC. The cost‐effectiveness of induction immunosuppression in kidney transplantation. Nephrology Dialysis Transplantation 2009;24(7):2258‐69. [MEDLINE: ] - PubMed
OPTN/SRTR 2008
-
- Rockville M, Richmond V. Annual Report of the US Scientific Registry of Transplant Recipients and the Organ Procurement and transplantation Network: Transplant data 1989‐1998. 2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998‐2007 http://optn.transplant.hrsa.gov/data/annualReport.asp. Rockville, MD, USA: U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2008.
Pascual 2001
-
- Pascual J, Marcen R, Ortuno J. Anti‐interleukin‐2 receptor antibodies: basiliximab and daclizumab. Nephrology Dialysis Transplantation 2001;16(9):1756‐60. [MEDLINE: ] - PubMed
Renal Group 2009
-
- Willis NS, Mitchell R, Higgins GY, Webster AC, Craig JC. Cochrane Renal Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2009, Issue 4. Art. No.: RENAL (accessed November 2009).
UK National Transplant Database 2009
-
- Transplant Activity report 2008‐2009, section 3 Kidney Activity. NHS Blood and Transplant, Transplant Activity in the UK. available at: http://www.organdonation.nhs.uk/ukt/statistics/statistics.jsp.
UK Renal Registry report 2007
-
- Ravanan R, Udayaraj U, Steenkamp R, Ansel D, Tomson C. Chapter 11: Measures of Care in Adult Renal Transplant Recipients in the UK. In: Ansell D, Feehally J, Feest T, Tomson C, Williams AJ, Warwick G editor(s). The Renal Association UK Renal Registry Tenth Annual Report. Bristol, UK: The Renal Association, December 2007.
Vanrenterghem 2001
-
- Vanrenterghem Y. Tailoring immunosuppressive therapy for renal transplant recipients. Pediatric Transplantation 2001;5(6):467‐72. [MEDLINE: ] - PubMed
References to other published versions of this review
Webster 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

