Antiviral treatment for chronic hepatitis C in patients with human immunodeficiency virus
- PMID: 20091566
- PMCID: PMC12327554
- DOI: 10.1002/14651858.CD004888.pub2
Antiviral treatment for chronic hepatitis C in patients with human immunodeficiency virus
Abstract
Background: Antiviral treatment for chronic hepatitis C may be less effective if patients are co-infected with human immunodeficiency virus (HIV).
Objectives: To assess the benefits and harms of antiviral treatment for chronic hepatitis C in patients with HIV.
Search strategy: Trials were identified through manual and electronic searches in The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded. The last search was May 2009.
Selection criteria: Randomised trials comparing at least 12 weeks of any anti-HCV treatment versus another treatment regimen or no treatment. Included patients had chronic hepatitis C and stable HIV irrespective of previous antiviral therapy.
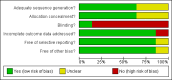
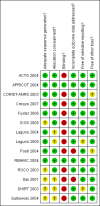
Data collection and analysis: Data extraction and assessment of risk of bias were done in duplicate. Analysis was by intention-to-treat.
Main results: Fourteen trials were included. None of the included 2269 patients were previously treated for chronic hepatitis C. Peginterferon (either 2a, 180 microgram, or 2b, 1.5 microgram/kg, once weekly) plus ribavirin was more effective in achieving end of treatment and sustained virological response compared with interferon plus ribavirin (5 trials, 1340 patients) or peginterferon (2 trials, 714 patients). The benefit of peginterferon plus ribavirin was seen irrespective of HCV genotype although patients with genotype 1 or 4 had lower response rates (27%) than patients with genotype 2 or 3 (56%). The remaining trials compared different treatment regimens in patients who were treatment naive or had no virological response after three months of treatment, but overall they had not enough power to show any effect of increasing the dose of interferon or adding both amantadine or ribavirin. The overall mortality was 23/2111 patients with no significant differences between treatment regimens. Treatment increased the risk of adverse events including anaemia and flu-like symptoms, and several serious adverse events occurred including fatal lactic acidosis, liver failure, and suicide due to depression.
Authors' conclusions: Peginterferon plus ribavirin may be considered a treatment for patients with chronic hepatitis C and stable HIV who have not received treatment for hepatitis C as the intervention may clear the blood of HCV RNA. Supporting evidence comes mainly from the analysis of this non-validated surrogate outcome assessed in comparisons against other antiviral treatments. There is no evidence on treatment of patients who have relapsed or did not respond to previous therapy. Careful monitoring of adverse events is warranted.
Conflict of interest statement
None of the authors have potential conflicts of interest regarding the present review.
Figures













Update of
References
References to studies included in this review
ACTG 2004 {published data only}
-
- Chung R, Andersen J, Alston B, Vallee M, Robbins G, Nevin T, et al. A randomized, controlled trial of pegylated interferon alpha‐2a with ribavirin vs. interferon alpha‐2a with ribavirin for the treatment of chronic HCV in HIV co‐infection: ACTG A5071. 9th Conference on Retroviruses and Opportunistic Infections (CROI). 2002:LB15.
-
- Chung R, Andersen J, Volberding P, Robbins R, Liu T, Sherman K, et al. A randomized, controlled trial of peg‐interferon‐alfa‐2a plus ribavirin vs interferon‐alfa‐2a plus ribavirin for chronic hepatitis C virus infection in HIV‐co‐infected persons: follow‐up results of ACTG A5071. The 11th Conference on Retroviruses and Opportunistic Infections. 2004:110.
-
- Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon alfa‐2a plus ribavirin versus interferon alfa‐2a plus ribavirin for chronic hepatitis C in HIV‐coinfected persons. New England Journal of Medicine 2004;351:451‐9. [ClinicalTrials.gov NCT00008463] - PMC - PubMed
APRICOT 2004 {published data only}
-
- Dore GJ, Torriani FJ, Rodriguez‐Torres M, Brau N, Sulkowski M, Lamoglia RS, et al. Baseline factors prognostic of sustained virological response in patients with HIV‐hepatitis C virus co‐infection. AIDS 2007;21:1555‐9. - PubMed
-
- Hornberger J, Torriani JF, Dieterich DT, Brau N, Sulkowski MS, Rodriguez‐Torres M, et al. Cost‐effectiveness of peginterferon alfa‐2a (40 kDa) plus ribavirin in patients with HIV and hepatitis C virus co‐infection. Journal of Clinical Virology 2006;36:283‐91. - PubMed
-
- Lissen E, Clumeck N, Sola, R, Mendes‐Correa M, Montaner J, Nelson M, et al. Histological response to pegIFNalpha‐2a (40KD) plus ribavirin in HIV‐hepatitis C virus co‐infection. AIDS 2006;20:2175‐81. - PubMed
-
- Mauss S, Valenti W, DePamphilis J, Duff F, Cupelli L, Passe S, et al. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon‐based therapy. AIDS 2004;18:F21‐F25. - PubMed
-
- Opravil M, Sasadeusz J, Cooper DA, Rockstroh JK, Clumeck N, Clotet B, et al. Effect of baseline CD4 cell count on the efficacy and safety of peginterferon Alfa‐2a (40KD) plus ribavirin in patients with HIV/hepatitis C virus coinfection. Journal of Acquired Immune Deficiency Syndromes 2008;47:36‐49. - PubMed
CORIST‐ANRS 2003 {published data only}
-
- Salmon‐Ceron D, Lassalle R, Pruvost A, Benech H, Bouvier‐Alias M, Payan C, et al. Interferon‐ribavirin in association with stavudine has no impact on plasma human immunodeficiency virus (HIV) type 1 level in patients coinfected with HIV and hepatitis C virus: a CORIST‐ANRS HC1 trial. Clinical Infectious Diseases 2003;36:1295‐304. - PubMed
Crespo 2007 {published data only}
-
- Crespo M, Esteban J, Ribera E, Falco V, Sauleda S, Buti M, et al. Utility of week‐4 viral response to tailor treatment duration in hepatitis C virus genotype 3/HIV co‐infected patients. AIDS 2007;21:477‐81. - PubMed
-
- Crespo M, Sauleda S, Esteban JI, Juarez A, Ribera E, Andreu AL, et al. Peginterferon alpha‐2b plus ribavirin vs interferon alpha‐2b plus ribavirin for chronic hepatitis C in HIV‐coinfected patients. Journal of Viral Hepatitis 2007;14:228‐38. - PubMed
Fuster 2006 {published data only}
-
- Fuster D, Planas R, Gonzalez J, Force L, Cervantes M, Vilaró J, et al. Results of a study of prolonging treatment with pegylated interferon‐alpha2a plus ribavirin in HIV/HCV‐coinfected patients with no early virological response. Antiviral Therapy 2006;11:473‐82. - PubMed
ICOS 2005 {published data only}
-
- Cargnel A, Angeli E, Mainini A, Gubertini G, Giorgi R, Schiavini M, et al. Open, randomized, multicentre Italian trial on PEG‐IFN plus ribavirin versus PEG‐IFN monotherapy for chronic hepatitis C in HIV‐coinfected patients on HAART. Antiviral Therapy 2005;10:309‐17. - PubMed
Laguno 2004 {published data only}
-
- Laguno M, Larrousse M, Murillas J, Blanco JL, León A, Milinkovic A, et al. Predictive value of early virologic response in HIV/hepatitis C virus‐coinfected patients treated with an interferon‐based regimen plus ribavirin. Journal of Acquired Immune Deficiency Syndromes 2007;44:174‐8. - PubMed
-
- Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez Tapias JM, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for treatment of HIV/HCV co‐infected patients. AIDS 2004;18:27‐36. - PubMed
Laguno 2009 {published data only}
-
- Laguno M, Cifuentes C, Murillas J, Veloso S, Larrousse M, Payeras A. Randomized trial comparing pegylated interferon‐2b versus pegylated interferon‐2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology 2009;49(1):22‐31. - PubMed
Puoti 2004 {published data only}
RIBAVIC 2004 {published data only}
-
- Bani‐Sadr F, Carrat F, Bedossa P, Piroth L, Cacoub P, Perronne C, et al. Hepatic steatosis in HIV‐HCV coinfected patients: analysis of risk factors. AIDS 2006;20:525‐31. - PubMed
-
- Bani‐Sadr F, Carrat F, Pol S, Hor E, Rosenthal E, Goujard C, et al. Risk factors for symptomatics for symptomatic mithocondrial toxicity in HIV/hepatitis C virus‐coinfected patients during interferon plus ribavirin‐based therapy. Journal of Acquired Immune Deficiency Syndromes 2005;40:47‐52. - PubMed
-
- Bani‐Sadr F, Carrat F, Rosenthal E, Piroth L, Morand P, Lunel‐Fabiani F, et al. Spontaneous hepatic decompensation in patients coinfected with HIV and hepatitis C virus during interferon‐ribavirin combination treatment. Clinical Infectious Disesase 2005;41:1806‐9. - PubMed
-
- Bani‐Sadr F, Lapidus N, Melchior JC, Ravaux I, Bensalem M, Rosa I, et al. Severe weight loss in HIV / HCV‐coinfected patients treated with interferon plus ribavirin: incidence and risk factors. Journal of Viral Hepatitis 2008;15:255‐60. - PubMed
ROCO 2003 {published data only}
-
- Neau D, Galperine T, Legrand E, Pitard V, Neau‐Cransac M, Moreau JF, et al. T‐lymphocyte populations in hepatitis C and HIV co‐infected patients treated with interferon‐alfa‐2a and ribavirin. HIV Medicine 2003;4:120‐6. - PubMed
-
- Neau D, Jouvencel AC, Legrand E, Trimoulet P, Galperine T, Chitty I, et al. Hepatitis C virus genetic variability in 52 human immunodeficiency virus‐coinfected patients. Journal of Medical Virology 2003;71:41‐8. - PubMed
-
- Neau D, Trimoulet P, Winnock M, Rullier A, Bail B, Lacoste D, et al. Comparison of 2 regimens that include interferon‐alpha‐2a plus ribavirin for treatment of chronic hepatitis C in human immunodeficiency virus‐coinfected patients. Clinical Infectious Diseases 2003;36:1564‐71. - PubMed
Sax 2001 {published data only}
-
- Sax H, Friedel A, Renner E, Streuerwald MH, Weber R for the Swiss HIV cohort study. Pilot study of interferon alpha with and without amantadine for the treatment of hepatitis C in HIV coinfected individuals on antiretroviral therapy. Infection 2001;29:267‐70. - PubMed
SHIRT 2003 {published data only}
-
- Perez‐Olmeda M, Soriano V, Asensi V, Morales D, Romero M, Ochoa A, et al. Treatment of chronic hepatitis C in HIV‐infected patients with interferon alpha‐2b plus ribavirin. AIDS Research & Human Retroviruses 2003;19:1083‐9. - PubMed
Sulkowski 2004 {published data only}
-
- Sulkowski MS, Felizarta F, Smith C, Slim J, Berggren R, Goodman R, et al. Daily versus thrice‐weekly interferon alfa‐2b plus ribavirin for the treatment of chronic hepatitis C in HIV‐infected persons: a multicenter randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 2004;35:464‐72. - PubMed
References to studies excluded from this review
Brau 2004 {published data only}
-
- Brau N, Rodriguez‐Torres M, Prokupek D, Bonacini M, Giffen CA, Smith JJ, et al. Treatment of chronic hepatitis C in HIV/ HCV‐coinfection with interferon alpha‐2b+ full‐course vs. 16‐week delayed ribavirin. Hepatology 2004;39:989‐98. - PubMed
-
- Kostman J, Rodriguez‐Torres M, Prokupek D, Bonacini M, Brau N, Giffen C, et al. Characteristics of HCV/HIV coinfected persons enrolled into a multicenter randomized double‐blind placebo‐controlled trial of ribavirin/interferon alpha 2b combination therapy. International Conference AIDS 2000;13:A0428.
-
- Kostman JR, Rodriguez‐Torres M, Prokupek D, Bonacini M, Brau N, Hassanein T, et al. Results of a multicenter randomized double‐blind, controlled trial of interferon alpha‐2b/ribavirin combination therapy in HCV/HIV coinfected persons. http://www.amfar.org/binary‐data/AMFAR_PDF/pdf/7.pdf (accessed 23 September 2008).
-
- Kostman JR, Rodriguez‐Torres M, Prokupek D, Brau N, Bonacini M, Hassanein T, et al. Results of a multicentric randomized, double blind, controlled trial of interferon a‐2b/ribavirin combination therapy in HCV/HIV co‐infected persons. International AIDS Society Meetings 2006.
-
- Sulkowski MS. Defining the standard of care: randomized controlled trials for the treatment of hepatitis C in the HIV‐infected person. Hepatology 2004;39:906‐8. - PubMed
Coral 2007 {published data only}
-
- Tural C, Solà R, Rubio R, Santín M, Planas R, Quereda C, et al. Safety and efficacy of an induction dose of pegylated interferon alpha‐2a on early hepatitis C virus kinetics in HIV/HCV co‐infected patients: the CORAL‐1 multicentre pilot study. Journal of Viral Hepatitis 2007;14:704‐13. - PubMed
Khalili 2005 {published data only}
-
- Kahlili M, Bernestein D, Lentz E, Barylski D, Hoffman‐Terry M. Pegylated interferon alpha‐2a with or without ribavirin in HCV/HIV coinfection: partially blinded, randomized multicenter trial. Digestive Diseases and Sciences 2003;50:1148‐55. - PubMed
Makris 1991 {published data only}
-
- Makris M, Preston FE, Triger DR, Underwood JCE, Westlake L, Adelman MI. A randomized controlled trial of recombinant interferon‐α in chronic hepatitis C in Hemophiliacs. Blood 1991;78(1):1672‐7. - PubMed
References to ongoing studies
ANRSHC12 2003 {unpublished data only}
-
- Efficacy of pegylated interferon on liver fibrosis in co‐infected patient with HIV and C hepatitis who failed to active treatment for HCV. ANRSHC12 Fibrostop.. Ongoing study July 2003..
Hoffmann‐La Roche 2006 {unpublished data only}
-
- A randomized multicenter, double blinded, phase IV study comparing the safety and efficacy of pegasys 180 µg plus copegus 1000 or 1200 mg to the currently approved combination of pegasys 180 µg plus copegus 800 mg in interferon‐naïve patients with chronic hepatitis C genotype 1 virus infection coi.. Ongoing study June 2006..
HRN004 2005 {unpublished data only}
-
- A multi‐center, randomized, open‐label, phase IIIb study investigating the safety and efficacy of peginterferon a‐2a plus ribavirin for the treatment of chronic hepatitis C infection in HIV infected persons who have failed to achieve a sustained virologic response following previous interferon therapy.. Ongoing study September 2005..
HRN005 2005 {unpublished data only}
-
- PEG‐interferon a‐2b + ribavirin for treatment of chronic hepatitis C infection in HIV‐infected persons not previously treated with interferon.. Ongoing study September 2005..
NIAID 2004 {unpublished data only}
-
- A randomised controlled trial to evaluate the safety and efficacy of twice‐weekly peginterferon alfa 2a and ribavirin induction therapy for chronic hepatitis C in patients who are coinfected with HIV‐1.. Ongoing study June 2004..
Perico 2007 {unpublished data only}
-
- Open, multicentre, randomised phase IV trial to evaluate efficacy/safety to extend treatment duration with peginterferon alfa‐2a+high dose of ribavirin supporting erythropoietinin treatment of CHC in HIV‐HCV patients who not clear virus at week 4.. Ongoing study June 2007..
San Cecilio 2005 {unpublished data only}
-
- Open, randomised, multicenter phase IV study to evaluate efficacy and safety to extend treatment 24 weeks in co‐infected HIV‐HCV patients genotype 1 and/or 4.. Ongoing study October 2005..
Valencia 2008 {unpublished data only}
-
- Open, randomized and multicenter phase IV study to compare the efficacy and safety of two different treatments duration 24 versus 48 weeks in chronic hepatitis C genotypes 2 and/or 3 co‐infected HIV‐HCV patients.. Ongoing study November 2005..
Additional references
Alter 1992
-
- Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community‐acquired hepatitis C in the United States. The Sentinel Countries Chronic non‐A, non‐B Hepatitis Study Team. New England Journal of Medicine 1992;327(27):1899‐905. - PubMed
Benhamou 1999
-
- Benhamou Y, Bochet M, Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 2001;30:1054‐8. - PubMed
Bica 2001
-
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end‐stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseses 2001;32:492‐7. - PubMed
Brok 2005
Daar 2001
-
- Daar ES, Lynn H, Donfield S, Gomperts E, O'Brien SJ, Hilgartner MW, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. Journal of Infectious Diseases 2001;183:589‐95. - PubMed
Di Bisceglie 2008
Di Martino 2001
-
- Martino V, Rufat P, Boyer N, Renard P, Degos F, Martinot‐Peignoux M, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long‐term retrospective cohort study. Hepatology 2001;34(6):1193‐9. - PubMed
Dienstag 2006
-
- Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology 2006;130:225‐30. - PubMed
EACS guidelines
-
- Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV‐infected adults. HIV Medicine 2008;9:82‐8. - PubMed
Eyster 1994
-
- Eyster ME, Fried MW, Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood 1994;84(4):1020‐3. - PubMed
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163:493‐501. - PubMed
Gluud 2009
-
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2009, Issue 1. Art. No.: LIVER.
Graham 2001
-
- Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta‐analysis. Clinical Infectious Diseases 2001;33:562‐9. - PubMed
Greub 2000
-
- Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival and immune recovery during antiretroviral therapy in patients with HIV‐1 and HCV hepatitis C virus infection: the Swiss HIV Cohort study. Lancet 2000;356:1800‐5. - PubMed
Hadziyannis 2006
-
- Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Annals of Internal Medicine 2004;140:346‐55. - PubMed
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
Kim 2007
-
- Kim AI, Dorn A, Bouajram R, Saab S. The treatment of chronic hepatitis C in HIV‐infected patients: a meta‐analysis. HIV Medicine 2007;8:312‐21. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Marcellin 1994
-
- Marcellin P, Martinot‐Peignoux M, Elias A, Branger M, Courtois F, Level R, et al. Hepatitis C virus (HCV) viremia in human immunodeficiency virus‐seronegative and ‐seropositive patients with indeterminate HCV recombinant immunoblot assay. The Journal of Infectious Disease 1994;170(2):433‐5. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Mohsen 2002
Myers 2002
Olmelda 2003
-
- Perez‐Olmeda M, Soriano V, Asensi V, Morales D, Romero M, Ochoa A, et al. Treatment of chronic hepatitis C in HIV‐infected patients with interferon alpha‐2b plus ribavirin. AIDS Research and Human Retroviruses 2003;19:1083‐9. - PubMed
Pineda 2005
-
- Pineda JA, Romero‐Gómez M, Díaz‐García F, Girón‐González JA, Montero JL, Torre‐Cisneros J, et al. HIV coinfection shortens the survival of patients with hepatitis C virus‐related decompensated cirrhosis. Hepatology 2005;41(4):779‐89. - PubMed
Poynard 1996
-
- Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, et al. Meta‐analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996;24:778‐89. - PubMed
RevMan 2008 [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rockstroh 2004
-
- Rockstroh JK, Spengler U. HIV and hepatitis C virus co‐infection. The Lancet Infectious Diseases 2004;4:437‐44. - PubMed
Rockstroh 2005
-
- Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, et al. Influence of hepatitis C virus infection on HIV‐1 disease progression and response to highly active antiretroviral therapy. Journal of Infectious Diseases 2005;192:992‐1002. - PubMed
Rodriguez 2001
-
- Rodriguez‐Rosado R, Perez‐Olmeda M, Garcia‐Samaniego J, Soriano V. Management of hepatitis C in HIV‐infected persons. Antiviral Research 2001;52:189‐98. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Serfaty 2001
-
- Serfaty L, Costagliola D, Wendum D, Picard O, Meyohas MC, Girard PM, et al. Impact of early‐untreated HIV infection on chronic hepatitis C in intravenous drug users: a case‐control study. AIDS 2001;15(15):2011‐6. - PubMed
Simin 2007
-
- Simin M, Brok J, Stimac D, Gluud C, Gluud LL. Cochrane systematic review: pegylated interferon plus ribavirin vs. interferon plus ribavirin for chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2007;25:1153‐62. - PubMed
Soriano 2002
-
- Soriano V, Sulkowski M, Bergin C, Hatzakis A, Cacoub P, Katlama C, et al. Care of patients with chronic hepatitis C and HIV co‐infection: recommendations from the HIV‐HCV International Panel. AIDS 2002;16:813‐28. - PubMed
Soto 1997
-
- Soto B, Sánchez‐Quijano A, Rodrigo L, Olmo JA, García‐Bengoechea M, Hernández‐Quero J, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally‐acquired hepatitis C with an unusually rapid progression to cirrhosis. Journal of Hepatology 1997;26(1):1‐5. - PubMed
Thomas 2000
-
- Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host viral, and environmental factors. The Journal of American Medical Association 2000;284(4):450‐6. - PubMed
VA guidelines
-
- Yee HS, Currie SL, Darling JM, Wright TL. Management and treatment of hepatitis C viral infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center program and the National Hepatitis C Program office. The American Journal of Gastroenterology 2006;101:2360‐78. - PubMed
Villano 1999
-
- Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long‐term follow‐up after acute hepatitis C infection. Hepatology 1999;29(3):908‐14. - PubMed
Waldrep 2000
-
- Waldrep TW, Summers KK, Chiliade PA. Coinfection with HIV and HCV: more questions than answers?. Pharmacotherapy 2000;20:1499‐507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

