Amisulpride versus other atypical antipsychotics for schizophrenia
- PMID: 20091599
- PMCID: PMC4164462
- DOI: 10.1002/14651858.CD006624.pub2
Amisulpride versus other atypical antipsychotics for schizophrenia
Abstract
Background: In many countries of the industrialised world second generation (atypical) antipsychotics have become first line drug treatments for people with schizophrenia. The question as to whether, and if so how much, the effects of the various second generation antipsychotics differ is a matter of debate. In this review we examine how the efficacy and tolerability of amisulpride differs from that of other second generation antipsychotics.
Objectives: To evaluate the effects of amisulpride compared with other atypical antipsychotics for people with schizophrenia and schizophrenia-like psychoses.
Search strategy: We searched the Cochrane Schizophrenia Group Trials Register (April 2007) which is based on regular searches of BIOSIS, CINAHL, EMBASE, MEDLINE and PsycINFO.
Selection criteria: We included randomised, at least single-blind, trials comparing oral amisulpride with oral forms of aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone or zotepine in people with schizophrenia or schizophrenia-like psychoses.
Data collection and analysis: We extracted data independently. For continuous data we calculated weighted mean differences (MD), for dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) on an intention-to-treat basis based on a random effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate.
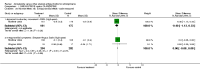
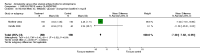
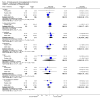
Main results: The review currently includes ten short to medium term trials with 1549 participants on three comparisons: amisulpride versus olanzapine, risperidone and ziprasidone. The overall attrition rate was considerable (34.7%) with no significant difference between groups. Amisulpride was similarly effective as olanzapine and risperidone and more effective than ziprasidone (leaving the study early due to inefficacy: n=123, 1 RCT, RR 0.21 CI 0.05 to 0.94, NNT 8 CI 5 to 50). Amisulpride induced less weight gain than risperidone (n=585, 3 RCTs, MD -0.99 CI -1.61 to -0.37) or olanzapine (n=671, 3 RCTs, MD -2.11 CI -2.94 to -1.29). Olanzapine was also associated with a higher increase of glucose (n=406, 2 RCTs, MD -7.30 CI -7.62 to -6.99). There was no difference in terms of cardiac effects and extra pyramidal symptoms (EPS) compared with olanzapine (akathisia: n= 587, 2 RCTs, RR 0.66 CI 0.36 to 1.21), compared with risperidone (akathisia: n=586, 3 RCTs, RR 0.80 CI 0.58 to 1.11) and compared with ziprasidone (akathisia: n=123, 1 RCT, RR 0.63, CI 0.11 to 3.67).
Authors' conclusions: There is little randomised evidence comparing amisulpride with other second generation antipsychotic drugs. We could only find trials comparing amisulpride with olanzapine, risperidone and ziprasidone. We found amisulpride may be somewhat more effective than ziprasidone, and more tolerable in terms of weight gain and other associated problems than olanzapine and risperidone. These data, however, are based on only ten short to medium term studies and therefore too limited to allow for firm conclusions.
Conflict of interest statement
Katja Komossa: none. Stefan Leucht: received speaker/consultancy honoria from Sanofi‐Aventis, BMS, Eli Lilly, Janssen, Lundbeck and Pfizer. He received research support from Sanofi‐Aventis and Eli Lilly. Werner Kissling: received speaker or consultancy honoraria from SanofiAventis, BMS, Lilly, Janssen, Lundbeck, Bayer and Pfizer. Christine Rummel: received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen‐Cilag, Eli Lilly and Pfizer. Heike Hunger: none. Franziska Schmid: none. Sandra Schwarz: none. Joaquim Silveira da Mota Neto: none.
Figures
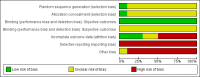



































































Update of
- doi: 10.1002/14651858.CD006624
Similar articles
-
Risperidone versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2011 Jan 19;2011(1):CD006626. doi: 10.1002/14651858.CD006626.pub2. Cochrane Database Syst Rev. 2011. PMID: 21249678 Free PMC article.
-
Olanzapine versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2010 Mar 17;2010(3):CD006654. doi: 10.1002/14651858.CD006654.pub2. Cochrane Database Syst Rev. 2010. PMID: 20238348 Free PMC article.
-
Ziprasidone versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD006627. doi: 10.1002/14651858.CD006627.pub2. Cochrane Database Syst Rev. 2009. PMID: 19821380 Free PMC article.
-
Clozapine versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2010 Nov 10;(11):CD006633. doi: 10.1002/14651858.CD006633.pub2. Cochrane Database Syst Rev. 2010. PMID: 21069690 Free PMC article.
-
Quetiapine versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2010 Jan 20;(1):CD006625. doi: 10.1002/14651858.CD006625.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Nov 18;(11):CD006625. doi: 10.1002/14651858.CD006625.pub3. PMID: 20091600 Free PMC article. Updated.
Cited by
-
Systematic Literature Review of the Methods Used to Compare Newer Second-Generation Agents for the Management of Schizophrenia: A focus on Health Technology Assessment.Pharmacoeconomics. 2015 Oct;33(10):1049-67. doi: 10.1007/s40273-015-0285-8. Pharmacoeconomics. 2015. PMID: 25963579
-
Second-Generation Antipsychotic Drugs for Patients with Schizophrenia: Systematic Literature Review and Meta-analysis of Metabolic and Cardiovascular Side Effects.Clin Drug Investig. 2021 Apr;41(4):303-319. doi: 10.1007/s40261-021-01000-1. Epub 2021 Mar 9. Clin Drug Investig. 2021. PMID: 33686614 Free PMC article.
-
Haloperidol (oral) versus olanzapine (oral) for people with schizophrenia and schizophrenia-spectrum disorders.Cochrane Database Syst Rev. 2024 Jul 3;7(7):CD013425. doi: 10.1002/14651858.CD013425.pub2. Cochrane Database Syst Rev. 2024. PMID: 38958149 Free PMC article.
-
Differential Effects of Aripiprazole and Amisulpride on Negative and Cognitive Symptoms in Patients With First-Episode Psychoses.Front Psychiatry. 2022 Mar 17;13:834333. doi: 10.3389/fpsyt.2022.834333. eCollection 2022. Front Psychiatry. 2022. PMID: 35370857 Free PMC article.
-
Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis.Can J Psychiatry. 2018 Jan 1;63(11):706743718777392. doi: 10.1177/0706743718777392. Online ahead of print. Can J Psychiatry. 2018. PMID: 29758999 Free PMC article.
References
References to studies included in this review
Bai 2005 {published data only}
-
- Bai YM, Ping LY, Lin CC, Wang YC, Liou YJ, Wu BJ, Chen TT, Chen JY, Lin CY, Chou P. Comparative effects of atypical antipsychotic on tardive dyskinesia and neurocognition: a 24‐week randomized, single‐blind, controlled study. Journal of the European College of Neuropsychopharmacology 2005;15(Suppl 3):S473.
Hwang 2003 {published data only}
-
- Hwang TJ, Lee S‐M, Sun HJ, Lin H‐N, Tsai S‐J, Lee Y‐C, Chen Y‐S. Amisulpride versus risperidone in the treatment of schizophrenic patients: a double‐blind pilot study in Taiwan. Journal of the Formosan Medical Association 2003;102(1):30‐6. - PubMed
Lecrubier 2006 {published data only}
-
- Lecrubier Y, Quintin P, Bouhassira M, Perrin E, Lancrenon S. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6‐month double‐blind controlled clinical trial. Acta Psychiatrica Scandinavica 2006;114:319‐27. - PubMed
Möller 2005 {published data only}
-
- Möller HJ, Riedel M, Eich FX. A randomised, double‐blind clinical trial comparing treatment with amisulpride or risperidone for six weeks in elderly patients with schizophrenia. Journal of the European College of Neuropsychopharmacology 2005;15(Suppl 3):S511.
Mortimer 2004 {published data only}
-
- Mortimer A. The European First Episode Schizophrenia Trial: comparison of outcome in first episode schizophrenia with different low dose antipsychotic regimens (EUFEST). National Research Register 2003; Vol. 1.
-
- Mortimer A, Martin S, Loo H, Peuskens J, SOLIANOL Sudy Group. A double‐blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. International Clinical Psychopharmacology 2004;19(2):63‐9. - PubMed
-
- Singh V. A six month international controlled trial of the therapeutic activity of amisulpride 200 to 800 mg/day verses olanzapine 5 to 20 mg/day in patients with schizophrenic disorders. National Research Register 2001; Vol. 1.
Olie 2006 {published data only}
-
- Olie J‐P, Spina E, Murray S, Yang R. Ziprasidone and amisulpride effectively treat negative symptoms of schizophrenia: results of a 12‐week, double‐blind study. International Clinical Psychopharmacology 2006;21(3):143‐51. - PubMed
-
- Olie JP, Spina E, Benattia I. Ziprasidone vs amisulpride for negative symptoms of schizophrenia. Journal of the European College of Neuropsychopharmacology 2002;12(Suppl 3):S313.
Peuskens 1999 {published data only}
-
- Peuskens J, Bech P, Möller H‐J, Bale R, Fleurot O, Rein W. Amisulpride vs. risperidone in the treatment of acute exacerations of schizophrenia. Psychiatry Research 1999;88:107‐17. - PubMed
Sechter 2002 {published data only}
-
- Nicholls CJ, Hale AS, Freemantle N. Cost‐effectiveness of amisulpride compared with risperidone in patients with schizophrenia. Journal of Drug Assessment 2003;6(2):79‐89.
-
- Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6‐month double‐blind study. Neuropsychopharmacology 2002;27:1071‐81. - PubMed
Vanelle 2006 {published data only}
-
- Vanelle JM, Douki S. A double‐blind randomised comparative trial of amisulpride versus olanzapine for 2 months in the treatment of subjects with schizophrenia and comorbid depression. European Psychiatry 2006;21:523‐30. - PubMed
-
- Vanelle JM, Douki S. Metabolic control in patients with comorbid schizophrenia and depression treated with amisulpride or olanzapine. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl 3):S284.
Wagner 2005 {published data only}
-
- Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kuhn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biological Psychiatry 2006;59(6):536‐45. - PubMed
-
- Wagner M, Quednow BB, Westheide J, Schlaepfer TE, Maier W, Kuhn K‐U. Cognitive improvement in schizophrenic patients does not require a serotonergic mechanism: randomized controlled trial of olanzapine vs amisulpride. Neuropsychopharmacology 2005;30(2):381‐90. - PubMed
References to studies excluded from this review
Baloescu 2006 {published data only}
-
- Baloescu A, Vasile D, Gheorghe MD, Grigorescu G. Side effects of atypical antipsychotics ‐ prediction factor for compliance. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S403.
Beuzen 2005 {published data only}
-
- Beuzen JN, Pans M, Modell S, Hagens P, McQuade R, Iwamoto T, Carson W. Naturalistic study of aripiprazole treatment. Proceedings of the XIII World Congress of Psychiatry; 2005 Sept 10‐15th; Cairo, Egypt. 2005.
Fleischhacker 2005 {published data only}
-
- Fleischhacker WW, Keet IPM, Kahn RS. The European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trial. Schizophrenia Research 2005;78(2‐3):147‐56. - PubMed
Giudicelli 1999 {published data only}
-
- Giudicelli A, Rein W. Social adaptation in schizophrenic patients: experience with amisulpride. Journal of the European College of Neuropsychopharmacology 1999;9:S275.
Kelemen 2006 {published data only}
-
- Kelemen O, Nagy O, Máttyássy A, Kiss I, Janka Z, Kéri S. Do second‐generation antipsychotics disrupt decision‐making abilities in schizophrenia?. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S430.
Möller 1997 {published data only}
-
- Möller HJ. Amisulpride A non conventional antipsychotic. Proceedings of the 6th World Congress of Biological Psychiatry; 1997 Jun 22‐27; Nice, France. 1997.
Moritz 2002 {published data only}
-
- Moritz S, Andresen B, Perro C, PERSIST Study Group, Schickel M, Krausz M, Naber D. Neurocognitive performance in first‐episode and chronic schizophrenic patients. European Archives of Psychiatry and Clinical Neuroscience 2002;252:33‐37. - PubMed
Olié 2001 {published data only}
-
- Olié JP, Stalla‐Bourdillon A. Social functioning and quality of life in schizophrenia: experience with amisulpride. European Neuropsychopharmacology 2001;11(3):261.
Oliveira‐Castro 1991 {published data only}
-
- Oliveira‐Castro J, Duval F, Makrani MC, Crocq M, Macher JP. Neuroendocrine effects of typical and atypical antipsychotic agents in schizophrenic patients [Effets neuroendocriniens d'agents antipsychotiques typiques et atypiques chez des patients schizophrenes]. Encephale 1991;17(2):100.
Rettenbacher 2004 {published data only}
-
- Rettenbacher Ma, Baumgartner S, Ebenbichler C, Edlinger M, Hofer a, Hummer M, Kemmler G, Lechleitner M, Fleischhacker W. Alterations of glucose metabolism under treatment with clozapine vs. amisulpride. Schizophrenia Research 2004;67(1):191.
Ryu 2006 {published data only}
-
- Ryu SH, Jang WS, Cho EY, Kim SK, Lee DS, Hong KS. Association of leptin gene polymorphism with antipsychotic drug‐induced weight gain. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S419.
Yagdiran 2000 {published data only}
-
- Yagdiran O, Krausz M, =PERSIST. Depressive symptoms under atypical neuroleptic treatment in schizophrenia. Nervenarzt 2000;71(Suppl 1):S135‐S136.
References to studies awaiting assessment
Anon 2005 {published data only}
-
- Anon. Risperdal consta clinical protocol RIS‐SCH‐4055. Clinical Trial Agreement For Pharmaceutical Industry Sponsored Research In NHS Trust 2005.
Assion 2008 {published data only}
-
- Assion HJ, Reinbold H, Lemanski S, Basilowski M, Juckel G. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double‐blind, placebo‐controlled trial. Pharmacopsychiatry 2008;41(1):24‐8. [MEDLINE: ] - PubMed
Beuzen 2005a {published data only}
-
- Beuzen J‐N, Pans M, Modell S, Hagens P, McQuade R, Iwamato T, et al. Broad effectiveness trial with aripiprazole in Europe Eu‐Beta. Proceedings of the 13th Congress of the Association of European Psychiatrists; 2005 Apr 2‐6; Munich, Germany. 2005.
Beuzen 2006 {published data only}
-
- Beuzen J‐N, Schirr K, Pans M, Hagens P, Kostic D, Carson W, et al. Effectiveness of aripiprazole in a naturalistic setting: a European multicenter study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4‐10; Davos, Switzerland. 2006.
Bhowmick 2010 {published data only}
-
- Bhowmick S, Hazra A, Ghosh M. Amisulpride versus olanzapine in the treatment of schizophrenia in Indian patients: randomized controlled trial. Australian and New Zealand Journal of Psychiatry 2010;44(3):237‐42. [MEDLINE: ] - PubMed
Birchwood 2005 {published data only}
-
- Birchwood M. Untitled. Psychological Medicine 2005;35(1):152. - PubMed
Dazzan 2011 {published data only}
-
- Dazzan P. Optimization of treatment and management of schizophrenia in europe study ‐ double blind (amisulpride or olanzapine) ‐ optimise2. http://public.ukcrn.org.uk/ 2011.
DRKS00003603 {published data only}
-
- DRKS00003603. A randomized double‐blind controlled trial to assess the benefits of olanzapine and amisulpride combination treatment in acutely ill schizophrenia patients.‐combine. http://apps.who.int/ 2012. - PubMed
Eufest 2005 {published data only}
-
- Boter H. The European First Episode Schizophrenia Trial (EUFEST): comparison of outcome in first episode schizophrenia with different low dose antipsychotic drug regimens. http://www.controlled‐trials.com 2005.
-
- Boter H, Derks EM, Fleischhacker WW, Davidson M, Kahn RS, Grp ES. Generalizability of the results of efficacy trials in first‐episode schizophrenia: comparisons between subgroups of participants of the European First Episode Schizophrenia Trial (EUFEST). Journal of Clinical Psychiatry 2010;71(1):58‐65. [MEDLINE: ] - PubMed
-
- Boter H, Peuskens J, Libiger J, Fleischhacker WW, Davidson M, Galderisi S, et al. Effectiveness of antipsychotics in first‐episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophrenia Research 2009;115(2‐3):97‐103. [MEDLINE: ] - PubMed
-
- Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. "Cognitive effects of antipsychotic drugs in first‐episode schizophrenia and schizophreniform disorder: a randomized, open‐label clinical trial (EUFEST)": correction. American Journal of Psychiatry 2009;166(6):731. - PubMed
-
- Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. Cognitive effects of antipsychotic drugs in first‐episode schizophrenia and schizophreniform disorder: a randomized, open‐label clinical trial (EUFEST). American Journal of Psychiatry 2009;166(6):675‐82. [MEDLINE: ] - PubMed
Fleischhacker 2009 {published data only}
-
- Fleischhacker WW. Safety and tolerability of first and second generation antipsychotics. Proceedings of the World Psychiatric Association International congress; 2009 April 1‐4th; Florence Italy. 2009.
Genc 2007 {published data only}
-
- Genc Y, Taner E, Candansayar S. Comparison of clozapine‐amisulpride and clozapine‐quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single‐blind randomized study. Advances in Therapy 2007;24(1):1‐13. [MEDLINE: ] - PubMed
Goder 2008 {published data only}
-
- Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck‐Hirschner M, Serafin I, et al. Effects of olanzapine on slow wave sleep, sleep spindles and sleep‐related memory consolidation in schizophrenia. Pharmacopsychiatry 2008;3:92‐9. - PubMed
Haro 2009 {published data only}
-
- Haro J. Amisulpride and quetiapine for schizophrenia. Stanley Foundation Research Programs 2009.
ISRCTN68824876 {published data only}
-
- ISRCTN68824876. Amisulpride augmentation in clozapine‐unresponsive schizophrenia. http://www.controlled‐trials.com 2010.
Jones 2006 {published data only}
-
- Barnes T, Jones P, Dunn G, Hayhurst K, Drake R, Lewis S. Large effect of baseline treatment with long acting antipsychotic drugs on randomized treatment outcomes. Schizophrenia Research 2010;117(2‐3):380.
-
- Peluso MJ, Lewis SW, Barnes TRE, Jones PB. Extrapyramidal motor side‐effects of first‐ and second‐generation antipsychotic drugs. British Journal of Psychiatry 2012;200(5):387‐92. [MEDLINE: ] - PubMed
Joyce 2004 {published data only}
-
- Joyce E, Rein W, Fleurot O. Effect of amisulpride and olanzapine on neuropsychological performance in schizophrenic patients: a sub‐analysis of a double‐blind, randomized clinical trial. International Journal of Neuropsychopharmacology 2004;7(Suppl 2):S244. [MEDLINE: ]
Kim 2007 {published data only}
-
- Kim S‐W, Shin I‐S, Kim J‐M, Lee S‐H, Lee J‐H, Yoon B‐H, et al. Amisulpride versus risperidone in the treatment of depression in patients with schizophrenia: a randomized, open‐label, controlled trial. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2007;31(7):1504‐9. [MEDLINE: ] - PubMed
Kim 2007a {published data only}
-
- Kim SW, Yoon JS, Lee SH, Yang DS, Lee JH, Kim WJ, et al. Effectiveness of switching from risperidone to amisulpride in stabilized schizophrenic patients with depression. European Neuropsychopharmacology 2007;17(Suppl 4):S440.
Lecrubier 2004 {published data only}
-
- Lecrubier Y, Rein W, Ponsard C. A double‐blind, randomized clinical trial of amisulpride and olanzapine in acute schizophrenia: patient responder profile. International Journal of Neuropsychopharmacology 2004;7(Suppl 2):S409‐S10.
Lewis 2005 {published data only}
-
- Lewis S, Barnes T, Murray R, Davies L, Kerwin R, Taylor D, et al. First generation versus second generation (non‐clozapine) antipsychotic drugs versus clozapine in schizophrenia: the Cutlass trials. Neuropsychopharmacology 2005;30(Suppl 1):S31‐2.
Mahmoud 2011 {published data only}
-
- Mahmoud A, Hayhurst KP, Drake RJ, Lewis SW. Second generation antipsychotics improve sexual dysfunction in schizophrenia: A randomised controlled trial. http://www.hindawi.com/journals/sprt/2011/596898/ (accessed 14 April 2012). [DOI: 10.1155/2011/596898] - DOI - PMC - PubMed
Martins 2002 {published data only}
-
- Peuskens J. Less weight gain with amisulpride: results from double‐blind studies vs. risperidone and olanzapine. Proceedings of the 16th European College of Neuropsychopharmacology Congress; 2003 Sep 20‐24; Prague, Czech Republic. 2003.
-
- Peuskens J, Hert M, Mortimer A, SOLIANOL SG. Metabolic control in patients with schizophrenia treated with amisulpride or olanzapine. International Clinical Psychopharmacology 2007;22(3):145‐52. [MEDLINE: ] - PubMed
McCue 2007 {published data only}
-
- McCue RE. Clozapine improves symptoms but not quality of life compared with other second‐generation antipsychotics in people with treatment resistant schizophrenia. Evidence‐Based Mental Health 2007;10(2):57. [MEDLINE: ] - PubMed
Moore 2006 {published data only}
-
- Moore N. Switching to aripiprazole from other second‐generation antipsychotics. Australian New Zealand Clinical Trials Registry 2006.
Mortimer 2007 {published data only}
-
- Mortimer AM, Joyce E, Balasubramaniam K, Choudhary PC, Saleem PT. Treatment with amisulpride and olanzapine improve neuropsychological function in schizophrenia. Human Psychopharmacology 2007;22(7):445‐54. [MEDLINE: ] - PubMed
NCT00419653 {published data only}
-
- NCT00419653. Modulation of regional brain activation in schizophrenic patients by pharmacological therapy with amisulpride, olanzapine or haloperidol a study with functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI). http://www.clinicaltrials.gov 2007.
NCT00761670 {published data only}
-
- NCT00761670. Efficacy study on cognitive functions in schizophrenic patients. http://www.clinicaltrials.gov 2008.
NCT00926965 {published data only}
-
- NCT00926965. Tardive dyskinesia and cognitive function. http://www.clinicaltrials.gov 2008.
NCT00956189 {published data only}
-
- NCT00956189. Identification and treatment response prediction of antipsychotic‐related metabolic syndrome. http://www.clinicaltrials.gov 2009.
NCT01029769 {published data only}
-
- NCT01029769. The switch study ‐ efficacy of an early antipsychotic switch in case of poor initial response to the treatment of schizophrenia. http://www.clinicaltrials.gov 2009.
NCT01246232 {published data only}
-
- Barnes T. Amisulpride augmentation in clozapine‐unresponsive schizophrenia ‐ AMICUS. http://public.ukcrn.org.uk 2011. - PMC - PubMed
-
- NCT01246232. Amisulpride augmentation in clozapine‐unresponsive schizophrenia. http://ClinicalTrials.gov/show/NCT01246232 2010.
NCT01446328 {published data only}
-
- NCT01446328. Bergen psychosis project 2. http://ClinicalTrials.gov/show/NCT01446328 2011.
NCT01448499 {published data only}
-
- NCT01448499. Clozapine versus amisulpride in treatment‐resistant schizophrenia patients. http://ClinicalTrials.gov/show/NCT01448499 2011.
NCT01609153 {published data only}
-
- NCT01609153. Is an antipsychotic combination treatment of olanzapine and amisulpride more effective than monotherapy. http://ClinicalTrials.gov/show/NCT01609153 2012.
Nicholls 2002 {published data only}
-
- Nicholls CJ, Hale AS, Freemantle N. Cost‐effectiveness of amisulpride compared with risperidone in patients with schizophrenia. Journal of Medical Economics 2002;6:31‐41.
Pfizer 2006 {published data only}
-
- Pfizer. Down‐titration schedules of amisulpride in patients suffering from schizophrenia or schizoaffective disorders when initiating therapy with ziprasidone: an open‐label, three‐months study. http://www.clinicalstudyresults.org/ 2006.
Rettenbacher 2007 {published data only}
-
- Rettenbacher MA, Hummer M, Hofer A, Baumgartner S, Ebenbichler C, Edlinger M, et al. Alterations of glucose metabolism during treatment with clozapine or amisulpride: results from a prospective 16‐week study. Journal of Psychopharmacology 2007;21(4):400‐4. [MEDLINE: ] - PubMed
Riedel 2009 {published data only}
-
- Riedel M, Eich FX, Moller HJ. A pilot study of the safety and efficacy of amisulpride and risperidone in elderly psychotic patients. European Psychiatry 2009;24(3):149‐53. - PubMed
Ruiz‐Doblado 2010 {published data only}
-
- Ruiz‐Doblado S, Baena‐Baldomero A, Esparrago‐Llorca G. Pharmacological augmentation strategies in clozapine‐resistant schizophrenia: Overcoming the resistance. Psiquiatria Biologica 2010;17(3):96‐101.
Schimmelmann 2005 {published data only}
-
- Schimmelmann BG, Moritz S, Karow A, Schafer I, Bussopulos A, Golks D, et al. Correlates of subjective well‐being in schizophrenic patients treated with atypical antipsychotics. International Journal of Psychiatry in Clinical Practice 2005;9(2):94‐8. - PubMed
Schmechtig 2010 {published data only}
-
- Schmechtig A, Dourish C, Craig K, Dawson GR, Williams S, Deakin W, et al. Effects of risperidone, amisulpride and nicotine on eye movement control and their modulation by high schizotypy. Society for Neuroscience Abstract Viewer and Itinerary Planner 2011;21:A99.
Schmechtig 2010a {published data only}
-
- Schmechtig A, Lees J, Dawson G, Dourish C, Craig K, Deakin B, et al. Effects of high schizotypy on control of eye movements: Modulation by antipsychotic drugs and nicotine. Proceedings of the 49th Annual meeting of the Americam College of Neuropsychopharmacology; 2010 Dec 5‐9; Miami, Florida. 2010.
Schmechtig 2011 {published data only}
-
- Schmechtig A, Lees J, Dawson GR, Dourish CT, Craig KJ, Deakin JFW, et al. Effects of risperidone, amisulpride and nicotine on eye movement control and their modulation by schizotypy. Pharmacopsychiatry. 2011:306. - PubMed
Sechter 2003 {published data only}
-
- Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y. Amisulpride vs risperidone in chronic schizophrenia: results of a 6‐month, double‐blind study: correction. Neuropsychopharmacology 2003;28(3):611. - PubMed
Shim 2012 {published data only}
Somers 2011 {published data only}
Vanelle 2004 {published data only}
-
- Vanelle J, Douki S. An 8‐week double blind, randomized trial comparing amisulpride and olanzapine in schizophrenic patients with depression. International Journal of Neuropsychopharmacology 2004;7(Suppl 2):S243.
Additional references
Altman 1996
Andreasen 1984
-
- Andreasen NC. Scale for the assessment of negative symptoms. University of Iowa, 1984.
APA 2004
-
- American Psychiatric Association. Practice guidelines for the treatment of patients with schizophrenia. 2nd Edition. American Psychiatric Publishing, Inc, 2004:1‐114.
Arnt 1998
-
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998;18:63‐101. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Carpenter 1984
-
- Heinrichs DW, Hanlon ET, Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10:388‐96. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town, South Africa. 2000.
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Duggan 2005
El‐Sayeh 2006
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Freeman 1997
-
- Freeman HL. Amisulpride compared with standard neuroleptics in acute exacerbations of schizophrenia: three efficacy studies. International clinical psychopharmacology 1997;12:11‐7. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. - PubMed
Gaebel 2006
-
- Gaebel W, Falkai P, Weinmann S, Wobrock T. [Behandlungsleitlinie Schizophrenie]. Treatment guidelines for schizophrenia. Steinkopf, 2006.
Goldman 1992
-
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM‐IV: a review of mesures of social functioning. American Journal of Psychiatry 1992;149:1148‐56. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy U. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health 1976.
Heres 2006
-
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry 2006;163:185‐94. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration 2008. Available from www.cochrane‐handbook.org.
Jones 2006 a
-
- Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life. Archives of General Psychiatry 2006;63:1079‐6. - PubMed
Kane 1988
-
- Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group. Clozapine for the treatment of treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45:789‐96. - PubMed
Kane 1993
-
- Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1993;46:585‐93. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Leucht 2002
-
- Leucht S, Pitschel‐Walz G, Engel RR, Kissling W. Amisulpride, an unusual "atypical" antipsychotic: A meta‐analysis of randomized controlled trials. American Journal of Psychiatry 2002;159:180‐90. - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. - PubMed
Liebermann 2005
-
- Liebermann JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marvaha S, Johnson S. Schizophrenia and employment ‐ a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT Statement: Revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐1. - PubMed
Mota Neto 2002
Möller 2000
-
- Möller HJ. New assessment of atypical antipsychotics [Aktuelle Bewertung neuer/atypischer Neuroleptika]. Nervenarzt 2000;71:329‐44. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Rein 1997
-
- Rein W, Turjanski S. Clinical update on amisulpride in deficit schizophrenia. International Clinical Psychopharmacology 1997;12:19‐27. - PubMed
Rust 1989
-
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989.
Simpson 1970
-
- Simpson EN, Angus JWF. A rating scale for extrapyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. - PubMed
Tandon 2008
-
- Tandon R, Keshavan MS, Nasralllah HA. Schizophrenia, "Just the facts" What we know in 2008. 2. Epidemiology and etiology. Schizophrenia research 2008;102:1‐18. - PubMed
Tsuang 1978
-
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐55. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. - PubMed
WHO 2001
-
- WHO. Mental Health: New Understanding, New Hope. The World Health report 2001.
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium, Sao Paulo, 23‐27 October 2007. 2007.
References to other published versions of this review
Leucht 2008
-
- Leucht S, Komossa K, Rumel‐Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. A Meta‐analysis of head‐to‐head comparisons of second generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry 2009;166(2):152‐63. [DOI: 10.1176/appi.ajp.2008.08030368] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

