Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events
- PMID: 20091646
- PMCID: PMC4015852
- DOI: 10.1002/14651858.CD007694.pub2
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events
Update in
-
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2021 Apr 14;4(4):CD007694. doi: 10.1002/14651858.CD007694.pub3. Cochrane Database Syst Rev. 2021. PMID: 33852162 Free PMC article.
Abstract
Background: An increase in serious adverse events with both regular formoterol and regular salmeterol in chronic asthma has been demonstrated in comparison with placebo in previous Cochrane reviews. This increase was significant in trials that did not randomise participants to an inhaled corticosteroid, but less certain in the smaller numbers of participants in trials that included an inhaled corticosteroid in the randomised treatment regimen.
Objectives: We set out to compare the risks of mortality and non-fatal serious adverse events in trials which have randomised patients with chronic asthma to regular formoterol versus regular salmeterol, when each are used with an inhaled corticosteroid as part of the randomised treatment.
Search strategy: Trials were identified using the Cochrane Airways Group Specialised Register of trials. Manufacturers' web sites of clinical trial registers were checked for unpublished trial data and Food and Drug Administration (FDA) submissions in relation to formoterol and salmeterol were also checked. The date of the most recent search was July 2009.
Selection criteria: Controlled clinical trials with a parallel design, recruiting patients of any age and severity of asthma were included if they randomised patients to treatment with regular formoterol versus regular salmeterol (each with a randomised inhaled corticosteroid), and were of at least 12 weeks duration.
Data collection and analysis: Two authors independently selected trials for inclusion in the review and extracted outcome data. Unpublished data on mortality and serious adverse events were sought from the sponsors and authors.
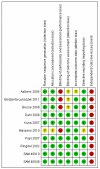
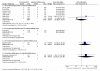
Main results: Eight studies met the eligibility criteria of the review recruiting 6,163 adults and adolescents. There were seven studies (involving 5,935 adults and adolescents) comparing formoterol and budesonide to salmeterol and fluticasone. All but one study administered the products as a combined inhaler, and most used formoterol 50 mcg and budesonide 400 mcg twice daily versus salmeterol 50 mcg and fluticasone 250 mcg twice daily. There were two deaths overall (one on each combination) and neither were thought to be related to asthma.There was no significant difference between treatment groups for non-fatal serious adverse events, either all-cause (Peto OR 1.14; 95% CI 0.82 to 1.59, I(2) = 26%) or asthma-related (Peto OR 0.69; 95% CI 0.37 to 1.26, I(2) = 33%). Over 23 weeks the rates for all-cause serious adverse events were 2.6% on formoterol and budesonide and 2.3% on salmeterol and fluticasone, and for asthma-related serious adverse events, 0.6% and 0.8% respectively.There was one study (228 adults) comparing formoterol and beclomethasone to salmeterol and fluticasone, but there were no deaths or hospital admissions.No studies were found in children.
Authors' conclusions: The seven identified studies in adults did not show any significant difference in safety between formoterol and budesonide in comparison with salmeterol and fluticasone. Asthma-related serious adverse events were rare, and there were no reported asthma-related deaths. There was a single small study comparing formoterol and beclomethasone to salmeterol and fluticasone in adults, but no serious adverse events occurred in this study. No studies were found in children.Overall there is insufficient evidence to decide whether regular formoterol and budesonide or beclomethasone have equivalent or different safety profiles from salmeterol and fluticasone.
Figures


Comment in
-
Asthma challenges: The place of inhaled long-acting beta-agonists.Cochrane Database Syst Rev. 2010 Apr 14;2011(4):ED000002. doi: 10.1002/14651858.ED000002. Cochrane Database Syst Rev. 2010. PMID: 21833925 Free PMC article. No abstract available.
References
References to studies included in this review
-
- Aalbers R. Fixed or adjustable maintenance-dose budesonide/formoterol compared with fixed maintenance-dose salmeterol fluticasone propionate in asthma patients aged [greater-than or equal to]16 years: post hoc analysis of a randomized, double-blind open-label extension, parallel-group study. Clinical Drug Investigation. 2010;Vol. 30(issue 7):439–51. - PubMed
-
- Aalbers R, Backer V, Kava TT, Welte T, Omenaas ER, Bergqvist PBF, et al. Adjustable dosing with budesonide/formoterol reduces the rate of asthma exacerbations compared with fixed dosing salmeterol/fluticasone. European Respiratory Society. 2003:2–20.
-
- Aalbers R, Backer V, Kava TTK, Omenaas ER, Sandstrom T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research & Opinion. 2004;20(2):225–40. - PubMed
-
- Aalbers R, Harris A, Naya I. Adjustable dosing with budesonide/formoterol achieves sustained guideline ‘well-controlled asthma’ following step down in treatment. European Respiratory Journal. 2005;26(Suppl 49):50s.
-
- Aalbers R, Welte T, Jorup C. Adjustable maintenance dosing (AMD) with budesonide /formoterol (B/F) meets guideline-defined management goals more effectively than fixed dosing (FD) with B/F or salmeterol/fluticasone (S/FL) [Abstract] European Respiratory Journal. 2004;Vol. 24(issue Suppl 48):311s.
-
- AstraZeneca (SD-039-0686) [Accessed 19 October 2007];A randomized, double-dummy, double-blind/open, parallel-group, phase-III, multicentre, 7-month study to assess the efficacy and safety of Symbicort® Turbuhaler® (budesonide/formoterol; 160/4.5 mcg delivered dose) given either as standard therapy (2 inhalations bid) or with an adjustable dosing regimen (1, 2 or 4 inhalations bid) versus Seretide™ Diskus™ (salmeterol/fluticasone; 50/250 mcg metered dose) given as standard therapy (1 inhalation bid) in adult and adolescent asthmatic patients. AstraZeneca Clinical Trials Register issue. http://www.astrazenecaclinicaltrials.com/Article/512577.aspx.
-
- Welte T, Aalbers R, Naya I. Budesonide/formoterol adjustable maintenance dosing (B/F AMD) reduces the burden of asthma more effectively than fixed dosing (FD) with B/F or salmeterol/fluticasone (S/FL) [Abstract] European Respiratory Journal. 2004;Vol. 24(issue Suppl 48):508s.
-
- Bodzenta-Lukaszyk A, Dymek A, Mansikka H. Fluticasone propionate/formoterol fumarate combination therapy has a more rapid onset of action than fluticasone propionate/salmeterol xinafoate in the treatment of asthma: a randomised controlled trial [Abstract] Thorax. 2010;Vol. 65(issue Suppl 4):P24.
-
- Bodzenta-Lukaszyk A, Dymek A, Mansikka H. Fluticasone propionate/formoterol fumarate combination therapy is as effective as fluticasone propionate/salmeterol xinafoate in the treatment of asthma: a randomised controlled trial [Abstract] Thorax. 2010;Vol. 65(issue Suppl 4):P177.
-
- Bodzenta-Lukaszyk A, Dymek A, McAulay K, Mansikka H. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulmonary Medicine. 2011;Vol. 11:28. - PMC - PubMed
-
- Ambrose H, Lawrance R, Goldman M. Beta-adrenergic receptor gly16arg variation: effect on response to budesonide/formoterol or fluticasone propionate/salmeterol in asthma patients. Chest. 2007;Vol. 132(issue 4):478a.
-
- AstraZeneca (D5896C00005) [accessed 13 March 2008];A two-stage randomized, open-label, parallel group, phase III, multicenter, 7 month study to assess the efficacy and safety of Symbicort pMDI administered either as fixed or as an adjustable regimen versus a fixed regimen of Advair in subjects 12 years of age and older with asthma. AstraZeneca Clinical Trials Register. 2006 http://www.astrazenecaclinicaltrials.com.
-
- Busse WW, Shah SR, Somerville L, Martin P, Goldman M. [accessed 13 March 2008];Comparison of asthma exacerbations and lung function with adjustable-dose budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI), fixed-dose BUD/FM pMDI, and fixed-dose fluticasone/salmeterol dry powder inhaler (FP/SM DPI) 2007 :A191. http://www.abstracts2view.com/ats07.
-
- Busse WW, Shah SR, Somerville L, Parasuraman B, Martin P, Goldman M. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. Journal of Allergy & Clinical Immunology. 2008;121(6):1407–14. - PubMed
-
- Shah SR, Busse WW, McElhattan J, O’Brien CD, Goldman M. Efficacy and tolerability of fixed-dose (FD) and adjustable-dose (AD) budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI) and FD fluticasone propionate/salmeterol dry powder inhaler (FP/SAL DPI) within racial groups [Abstract] Journal of Allergy and Clinical Immunology. 2009;Vol. 123(issue 2 Suppl 1):S80.
-
- Shah SR, Busse WW, Somerville L, Martin P, Goldman M. Asthma control with adjustable-and fixed-dose budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI) and fixed-dose fluticasone/salmeterol dry powder inhaler (FP/SM DPI [Abstract]. American Thoracic Society International Conference; San Francisco, California, USA. May 18-23, 2007; 2007. Poster #K4.
-
- Somerville L, Busse WW, Shah SR, Martin P, Goldman M. Safety of adjustable-dose budesonide (BUD)/formoterol (FM) pressurized metered-dose inhaler (pMDI), fixed-dose (BUD/FM pMDI and fixed-dose fluticasone (FP)/salmeterol (SM) dry powder inhaler (DPI) in asthma patients [Abstract]. American Thoracic Society International Conference; San Francisco, California, USA. May 18-23, 2007; 2007; Poster #K3.
-
- Dahl R, Chuchalin A, Gor D, Yoxall S, Sharma R. EXCEL: A randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respiratory Medicine. 2006;Vol. 100(issue 7):1152–62. - PubMed
-
- Dahl R, Chuchalin A, Lindberg A, Jones M, Aggarwal K, Gor D. EXCEL regular maintenance therapy with salmeterol/fluticasone propionate combination (SFC) reduces exacerbations more effectively than the formoterol/budesonide combination (FBC) European Respiratory Journal. 2004;24(Suppl 48):309s.
-
- Dahl R, Chuchalin A, Ringdal N, Gor D, Jones M. Salmeterol/fluticasone (SFC) reduces moderate/severe exacerbations more effectively than formoterol/budesonide (FBC) with sustained maintenance therapy EXCEL [Abstract]. American Thoracic Society 2005 International Conference; San Diego, California. May 20-25; 2005. [C33] [Poster: F68]
-
- GlaxoSmithKline (SAM40040) [accessed 19 October 2007];A twenty-four week, randomised, double-dummy, double-blind, parallel group study to compare the rate of asthma exacerbations between SERETIDE DISKUS 50/250ìg 1 inhalation bd and formoterol/budesonide Breath-Actuated Dry Powder Inhaler (BADPI) 4.5/160ìg 2 inhalations bd in subjects with moderate to severe asthma. GlaxoSmithKline Clinical Trials Register issue. http://ctr.gsk.co.uk/Summary/fluticasone.salmeterol/IV.SAM40040.pdf.
-
- AstraZeneca (SD-0390735) [accessed 30 April 30 2009];Comparison of the efficacy and safety of one inhalation of Symbicort®Turbuhaler® 160/4.5 μg bid plus as-needed with two inhalations of SeretideTM EvohalerTM 25/125 μg bid plus Terbutaline Turbuhaler® 0.4 mg as-needed, and one inhalation of Symbicort® Turbuhaler® 320/9 μg bid plus Terbutaline Turbuhaler® 0.4 mg as-needed. A 6-month, randomised, double-blind, double-dummy, parallel-group, active-controlled,Multicentre, phase IIIB study in adult and adolescent asthmatic patients. http://www.astrazenecaclinicaltrials.com/.mshost2715844/content/content/....
-
- Bleecker ER, Postma DS, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta2-adrenergic receptor gene (ADRB2) on response to long-acting beta2-agonist (LABA) therapy. Journal Allergy and Clinical Immunology. 2007;119(2):523.
-
- Buhl R, Kuna P. Does the choice of ICS/LABA regimen influence exacerbation rates in asthma patients with high as needed use? [Abstract] European Respiratory Journal. 2007;Vol. 30(issue Suppl 51):617s. [P3620]
-
- Kuna P. Treatment comparison of budesonide formoterol with salmeterol fluticasone propionate in adults aged >=16 years with asthma: post hoc analysis of a randomized, double-blind study. Clinical Drug Investigation. 2010;Vol. 30(issue 9):565–79. - PubMed
-
- Kuna P, Peters J, Buhl R. Budesonide/formoterol as maintenance and reliever therapy reduces asthma exacerbations versus higher maintenance dose of budesonide/formoterol or salmeterol/fluticasone [Abstract] European Respiratory Journal. 2006;Vol. 28(issue Suppl 50):205s. [P1228]
-
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. International Journal of Clinical Practice. 2007;Vol. 61(issue 5):725–36. - PMC - PubMed
-
- Miller E, FitzGerald JM. Budesonide/formoterol as maintenance and reliever treatment compared to fixed dose combination strategies - a Canadian economic evaluation. Canadian Journal of Clinical Pharmacology/Journal Canadien de Pharmacologie Clinique. 2008;Vol. 15(issue 2):e165–76. - PubMed
-
- Price D, Wiren A, Kuna P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy: European Journal of Allergy and Clinical Immunology. 2007;Vol. 62(issue 10):1189–98. - PubMed
-
- Tamminen K, Laine J, Soini E, Martikainen J, Kankaanranta H. Cost-effectiveness analysis of budesonide/formoterol maintenance and reliever therapy versus fixed combination treatments for asthma in Finland. Current Medical Research and Opinion. 2008;24(12):3453–61. - PubMed
References to studies excluded from this review
-
- Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to long acting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. [see comment]. [Review] [33 refs]. Comment in. Lancet. 2008 Dec 22;370(9605):2075–6. - PubMed
-
- Lancet. 2008;Vol. 370(issue 9605):2118–25.
-
- Dhillon S, Keating GM. Beclometasone dipropionate/formoterol: in an HFA-propelled pressurised metered-dose inhaler. Drugs. 2006;Vol. 66(issue 11):1475–83. - PubMed
-
- Hampel FC, Martin P, Mezzanotte WS. Early bronchodilatory effects of budesonide/formoterol pMDI compared with fluticasone/salmeterol DPI and albuterol pMDI: 2 randomized controlled trials in adults with persistent asthma previously treated with inhaled corticosteroids. Journal of Asthma. 2008;Vol. 45(issue 4):265–72. - PubMed
-
- Jung KS, Uh ST, Lee YC, Shim JJ, Park SK, Williams AE, et al. Comparison of the clinical efficacy and safety of salmeterol/fluticasone propionate versus current care in the management of persistent asthma in Korea. Current Medical Research & Opinion. 2008;Vol. 24(issue 12):3571–82. - PubMed
-
- Lee DKC, Currie GP, Cockburtn WJ, Lipworth BJ. Comparison of budesonide/formoterol versus fluticasone/salmeterol combination inhalers in moderate persistent asthma [abstract]. American Thoracic Society 99th International Conference; 2003. p. D094. Poster 613.
Additional references
-
- Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. European Respiratory Journal. 2002;19:182–91. - PubMed
-
- Beach JR, Young CL, Stenton SC, Avery AJ, Walters EH, Hendrick DJ. A comparison of the speeds of action of salmeterol and salbutamol in reversing methacholine-induced bronchoconstriction. Pulmonary Pharmacology. 1992;5(2):133–5. - PubMed
-
- Cates CJ, Cates MJ, Lasserson TJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. 2008;(Issue 4) DOI: 10.1002/14651858.CD006923.pub2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

