Haemostatic drugs for traumatic brain injury
- PMID: 20091656
- PMCID: PMC4066582
- DOI: 10.1002/14651858.CD007877.pub2
Haemostatic drugs for traumatic brain injury
Update in
-
WITHDRAWN: Haemostatic drugs for traumatic brain injury.Cochrane Database Syst Rev. 2015 May 13;2015(5):CD007877. doi: 10.1002/14651858.CD007877.pub3. Cochrane Database Syst Rev. 2015. PMID: 25970597 Free PMC article.
Abstract
Background: Traumatic brain injury (TBI) is a leading cause of death and disability. Intracranial bleeding is a common complication of TBI, and intracranial bleeding can develop or worsen after hospital admission. Haemostatic drugs may reduce the occurrence or size of intracranial bleeds and consequently lower the morbidity and mortality associated with TBI.
Objectives: To assess the effects of haemostatic drugs on mortality, disability and thrombotic complications in patients with traumatic brain injury.
Search strategy: We searched the electronic databases: Cochrane Injuries Group Specialised Register (3 February 2009), CENTRAL (The Cochrane Library 2009, Issue 1), MEDLINE (1950 to Week 3 2009), PubMed (searched 3 February 2009 (last 180 days)), EMBASE (1980 to Week 4 2009), CINAHL (1982 to January 2009), ISI Web of Science: Science Citation Index Expanded (SCI-EXPANDED) (1970 to January 2009), ISI Web of Science: Conference Proceedings Citation Index - Science (CPCI-S) (1990 to January 2009).
Selection criteria: We included published and unpublished randomised controlled trials comparing haemostatic drugs (antifibrinolytics: aprotinin, tranexamic acid (TXA), aminocaproic acid or recombined activated factor VIIa (rFVIIa)) with placebo, no treatment, or other treatment in patients with acute traumatic brain injury.
Data collection and analysis: Two review authors independently examined all electronic records, and extracted the data. We judged that there was clinical heterogeneity between trials so we did not attempt to pool the results of the included trials. The results are reported separately.
Main results: We included two trials. One was a post-hoc analysis of 30 TBI patients from a randomised controlled trial of rFVIIa in blunt trauma patients. The risk ratio for mortality at 30 days was 0.64 (95% CI 0.25 to 1.63) for rFVIIa compared to placebo. This result should be considered with caution as the subgroup analysis was not pre-specified for the trial. The other trial evaluated the effect of rFVIIa in 97 TBI patients with evidence of intracerebral bleeding in a computed tomography (CT) scan. The corresponding risk ratio for mortality at the last follow up was 1.08 (95% CI 0.44 to 2.68). The quality of the reporting of both trials was poor so it was difficult to assess the risk of bias.
Authors' conclusions: There is no reliable evidence from randomised controlled trials to support the effectiveness of haemostatic drugs in reducing mortality or disability in patients with TBI. New randomised controlled trials assessing the effects of haemostatic drugs in TBI patients should be conducted. These trials should be large enough to detect clinically plausible treatment effects.
Figures
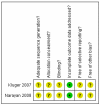

References
-
- Kluger Y, Riou B, Rossaint R, Rizoli SB, Boffard KD, Choong PI, Warren B, et al. Safety of rFVIIa in hemodynamically unstable polytrauma patients with traumatic brain injury: post hoc analysis of 30 patients from a prospective, randomized, placebo-controlled, double-blind clinical trial. Critical Care. 2007;11(4):R85. [published data only] - PMC - PubMed
-
- Narayan RK, Maas AI, Servadei F, Skolnick BE, Tillinger MN, Marshall LF. Progression of traumatic intracerebral hemorrhage: a prospective observational study. Journal of Neurotrauma. 2008;25(6):629–39. [published data only] - PubMed
References to studies excluded from this review
-
- Auer LM, Marth E, Heppner F, Holasek A. Proteolytic enzyme activity in patients with severe head injury and the effect of a proteinase inhibitor. Acta Neurochirurgica. 1979;49(3-4):207–17. [published data only] - PubMed
References to studies awaiting assessment
-
-
unpublished data only
-
References to ongoing studies
-
- CRASH-2 Ongoing study. May, 2005. [published data only]
-
- Tranexamic Acid for Preventing Progressive Intracranial Haemorrhage in Traumatic Brain Injury. Ongoing study. Oct, 2008. [published data only]
Additional references
-
- Astrup J. “Ultra-early” antifibrinolytic treatment of subarachnoidal bleeding with tranexamic acid. Ugeskrift for Laeger. 2006;168(11):1107–11. - PubMed
-
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized placebo controlled double blind clinical trials. Journal of Trauma. 2005;59:8–18. - PubMed
-
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58(Suppl(3)):25–46. discussion i-iv. - PubMed
-
- Ceylan S, Kuzeyli K, Ilbay K, Akturk F. Non operative management of acute extradural hematomas in children. Journal of Neurosurgical Science. 1992;36(2):85–8. - PubMed
-
- Coats T, Roberts IG, Shakur H. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database of Systematic Reviews. 2004;(Issue 4) [DOI: 10.1002/14651858.CD004896.pub2] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

