Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease
- PMID: 20091669
- PMCID: PMC2866264
- DOI: 10.1002/pro.340
Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease
Abstract
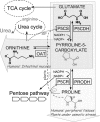
Pyrroline-5-carboxylate synthase (P5CS) is a bifunctional enzyme that exhibits glutamate kinase (GK) and gamma-glutamyl phosphate reductase (GPR) activities. The enzyme is highly relevant in humans because it belongs to a combined route for the interconversion of glutamate, ornithine and proline. The deficiency of P5CS activity in humans is associated with a rare, inherited metabolic disease. It is well established that some bacteria and plants accumulate proline in response to osmotic stress. The alignment of P5CSs from different species and analysis of the solved structures of GK and GPR reveal high sequence and structural conservation. The information acquired from different mutant enzymes with increased osmotolerant properties, together with the position of the insertion found in the longer human isoform, permit the delimitation of the regulatory site of GK and P5CS and the proposal of a model of P5CS architecture. Additionally, the GK moiety of the human enzyme has been modeled and the known clinical mutations and polymorphisms have been mapped.
Figures


References
-
- Baumgartner MR, Rabier D, Nassogne MC, Dufier JL, Padovani JP, Kamoun P, Valle D, Saudubray JM. Delta1-pyrroline-5-carboxylate synthase deficiency: neurodegeneration, cataracts and connective tissue manifestations combined with hyperammonaemia and reduced ornithine, citrulline, arginine and proline. Eur J Pediatr. 2005;164:31–36. - PubMed
-
- Hu CA, Lin WW, Obie C, Valle D. Molecular enzymology of mammalian delta1-pyrroline-5-carboxylate synthase. Alternative splice donor utilization generates isoforms with different sensitivity to ornithine inhibition. J Biol Chem. 1999;274:6754–6762. - PubMed
-
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

