NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae
- PMID: 20091781
- PMCID: PMC2967038
- DOI: 10.1002/glia.20968
NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae
Abstract
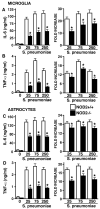
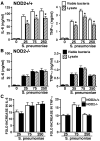
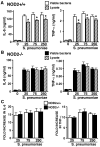
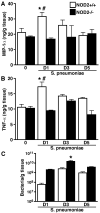
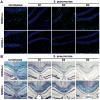
It is now widely accepted that resident central nervous system (CNS) cells such as microglia and astrocytes initiate and/or augment inflammation following trauma or infection. However, the mechanisms by which glial cells perceive microbial challenges are only now becoming apparent. We have recently demonstrated that microglia and astrocytes constitutively express nucleotide-binding oligomerization domain-2 (NOD2), a member of the novel nucleotide-binding domain leucine-rich repeat region-containing family of proteins (NLR) that functions as an intracellular receptor for a minimal motif present in all bacterial peptidoglycans. Furthermore, we have shown that this NLR is essential for glial responses to gram-negative pathogens and in vivo CNS inflammation elicited by these organisms. In the present study, we have established that intact Streptococcus pneumoniae, the major causative agent for gram-positive bacterial meningitis in adults, is a potent stimulus for the activation of the pivotal inflammatory transcription factor NF-kB and production of inflammatory cytokines in primary murine microglia and astrocytes. We demonstrate that NOD2 is essential for the maximal responses of these cells to intact S. pneumoniae but not cellular lysates. Finally, we have shown that this cytosolic pattern recognition receptor is required for the elevated inflammatory mediator levels, astrogliosis, and demyelination, following in vivo administration of this gram-positive CNS pathogen. As such, we suggest that NOD2 plays a critical role in the establishment of the lethal inflammation associated with streptococcal meningitis.
Figures
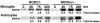
References
-
- Bergmann S, Lang A, Rohde M, Agarwal V, Rennemeier C, Grashoff C, Preissner KT, Hammerschmidt S. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J Cell Sci. 2009;122:256–267. - PubMed
-
- Bernatowicz A, Ködel U, Frei K, Fontana A, Pfister HW. Production of nitrite by primary rat astrocytes in response to pneumococci. J Neuroimmunol. 1995;60:53–61. - PubMed
-
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. - PubMed
-
- Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, Podschun R, Wruck CJ, Schröder JM, Pufe T, Lucius R. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol. 2008;67:1041–1054. - PubMed
-
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

