A common mechanism for adaptive scaling of reward and novelty
- PMID: 20091793
- PMCID: PMC3173863
- DOI: 10.1002/hbm.20939
A common mechanism for adaptive scaling of reward and novelty
Abstract
Declarative memory is remarkably adaptive in the way it maintains sensitivity to relative novelty in both unknown and highly familiar environments. However, the neural mechanisms underlying this contextual adaptation are poorly understood. On the basis of emerging links between novelty processing and reinforcement learning mechanisms, we hypothesized that responses to novelty will be adaptively scaled according to expected contextual probabilities of new and familiar events, in the same way that responses to prediction errors for rewards are scaled according to their expected range. Using functional magnetic resonance imaging in humans, we show that the influence of novelty and reward on memory formation in an incidental memory task is adaptively scaled and furthermore that the BOLD signal in orbital prefrontal and medial temporal cortices exhibits concomitant scaled adaptive coding. These findings demonstrate a new mechanism for adjusting gain and sensitivity in declarative memory in accordance with contextual probabilities and expectancies of future events.
Hum Brain Mapp, 2010. © 2010 Wiley-Liss, Inc.
Figures

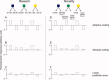
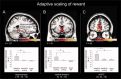
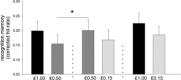

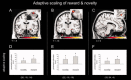
Similar articles
-
The novelty exploration bonus and its attentional modulation.Neuropsychologia. 2009 Sep;47(11):2272-81. doi: 10.1016/j.neuropsychologia.2009.01.015. Epub 2009 Jan 19. Neuropsychologia. 2009. PMID: 19524091
-
Contextual interaction between novelty and reward processing within the mesolimbic system.Hum Brain Mapp. 2012 Jun;33(6):1309-24. doi: 10.1002/hbm.21288. Epub 2011 Apr 21. Hum Brain Mapp. 2012. PMID: 21520353 Free PMC article.
-
The neural basis of novelty and appropriateness in processing of creative chunk decomposition.Neuroimage. 2015 Jun;113:122-32. doi: 10.1016/j.neuroimage.2015.03.030. Epub 2015 Mar 19. Neuroimage. 2015. PMID: 25797834
-
Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI.Neuroimage. 2011 Sep 15;58(2):647-55. doi: 10.1016/j.neuroimage.2011.06.038. Epub 2011 Jun 24. Neuroimage. 2011. PMID: 21723396
-
Age differences in the neural correlates of novelty processing: The effects of item-relatedness.Brain Res. 2015 Jul 1;1612:2-15. doi: 10.1016/j.brainres.2014.08.006. Epub 2014 Aug 19. Brain Res. 2015. PMID: 25149192 Review.
Cited by
-
Effects of positive and negative social feedback on motivation, evaluative learning, and socio-emotional processing.NPJ Sci Learn. 2023 Aug 16;8(1):28. doi: 10.1038/s41539-023-00178-7. NPJ Sci Learn. 2023. PMID: 37587116 Free PMC article.
-
Hippocampal networks habituate as novelty accumulates.Learn Mem. 2013 Mar 19;20(4):229-35. doi: 10.1101/lm.029728.112. Learn Mem. 2013. PMID: 23512939 Free PMC article.
-
Properties and temporal dynamics of choice- and action-predictive signals during item recognition decisions.Brain Struct Funct. 2020 Sep;225(7):2271-2286. doi: 10.1007/s00429-020-02124-4. Epub 2020 Aug 9. Brain Struct Funct. 2020. PMID: 32772167 Free PMC article.
-
Hedging your bets by learning reward correlations in the human brain.Neuron. 2011 Sep 22;71(6):1141-52. doi: 10.1016/j.neuron.2011.07.025. Epub 2011 Sep 21. Neuron. 2011. PMID: 21943609 Free PMC article.
-
Schemas, reinforcement learning and the medial prefrontal cortex.Nat Rev Neurosci. 2025 Mar;26(3):141-157. doi: 10.1038/s41583-024-00893-z. Epub 2025 Jan 7. Nat Rev Neurosci. 2025. PMID: 39775183 Review.
References
-
- Adcock RA,Thangavel A,Whitfield‐Gabrieli S,Knutson B,Gabrieli JD ( 2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50: 507–517. - PubMed
-
- Andersson JL,Hutton C,Ashburner J,Turner R,Friston K ( 2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–919. - PubMed
-
- Barlow HB ( 1961): Possible principles underlying the transformation of sensory messages In: Rosenblith WA, editor. Sensory Communication. Cambridge, MA: MIT Press; pp 217–234.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

