Fibrillar peptide gels in biotechnology and biomedicine
- PMID: 20091870
- PMCID: PMC3380801
- DOI: 10.1002/bip.21326
Fibrillar peptide gels in biotechnology and biomedicine
Abstract
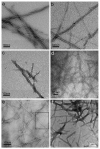
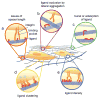
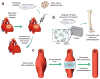
Peptides, peptidomimetics, and peptide derivatives that self-assemble into fibrillar gels have received increasing interest as synthetic extracellular matrices for applications in 3D cell culture and regenerative medicine. Recently, several of these fibrillizing molecules have been functionalized with bioactive components and chemical features such as cell-binding ligands, degradable sequences, drug eluting compounds, and cross-linkable groups, thereby producing gels that can reliably display multiple factors simultaneously. This capacity for incorporating precise levels of many different biological and chemical factors is advantageous given the natural complexity of cell-matrix interactions that many current biomaterial strategies seek to mimic. In this review, recent efforts in the area of fibril-forming peptide materials are described, and advantages of biomaterials containing multiple modular elements are outlined. In addition, a few hurdles and open questions surrounding fibrillar peptide gels are discussed, including issues of the materials' structural heterogeneity, challenges in fully characterizing the diversity of their self-assembled structures, and incomplete knowledge of how the materials are processed in vivo.
(c) 2010 Wiley Periodicals, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

