Culture human mesenchymal stem cells with calcium phosphate cement scaffolds for bone repair
- PMID: 20091907
- PMCID: PMC2989678
- DOI: 10.1002/jbm.b.31563
Culture human mesenchymal stem cells with calcium phosphate cement scaffolds for bone repair
Abstract
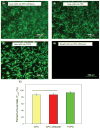
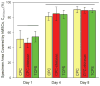
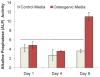
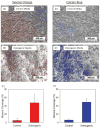
Because of its moldability and excellent osteoconductivity, calcium phosphate cement (CPC) is highly promising for craniofacial and orthopedic applications. The objectives of this study were to investigate the response of human mesenchymal stem cells (hMSCs) to a high-strength CPC-chitosan scaffold and to examine cell proliferation and osteogenic differentiation. hMSCs were seeded onto CPC-chitosan composite, CPC control, and tissue culture polystyrene (TCPS). Alkaline phosphatase activity (ALP) and mineralization of hMSCs were measured. CPC-chitosan had a flexural strength (mean + or - SD; n = 5) of (19.5 + or - 1.4) MPa, higher than (8.0 + or - 1.4) MPa of CPC control (p < 0.05). The percentage of live hMSCs on CPC-chitosan was (90.5 + or - 1.3)% at 8 days, matching (90.7 + or - 3.8)% of CPC control (p > 0.1). The CPC-chitosan surface area covered by the attached hMSCs increased from (51 + or - 11)% at 1 day to (90 + or - 4)% at 8 days (p < 0.05), matching those of CPC control (p > 0.1). Hence, the CPC strength was significantly increased via chitosan without compromising the hMSC response. At 8 days, there was a significant increase in ALP of cells in osteogenic media (10.99 + or - 0.93) [(mM pNpp/min)/(microg DNA)] versus control media (3.62 + or - 0.40) (p < 0.05). hMSCs in osteogenic media exhibited greater mineralization area of (47.5 + or - 19.7)% compared with (6.1 + or - 2.3)% in control medium on TCPS (p < 0.05). In conclusion, hMSCs showed excellent attachment and viability on the strong and tough CPC-chitosan scaffold, matching the hMSC response on CPC control. hMSCs were successfully differentiated down the osteogenic lineage. Hence, the strong, in situ hardening CPC-chitosan scaffold may be useful as a moderate load-bearing vehicle to deliver hMSCs for maxillofacial and orthopedic bone tissue engineering.
Figures


Similar articles
-
Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold.Biomaterials. 2009 May;30(14):2675-82. doi: 10.1016/j.biomaterials.2009.01.022. Epub 2009 Feb 1. Biomaterials. 2009. PMID: 19187958 Free PMC article.
-
Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair.Tissue Eng Part A. 2013 Apr;19(7-8):915-27. doi: 10.1089/ten.TEA.2012.0172. Epub 2013 Jan 28. Tissue Eng Part A. 2013. PMID: 23092172 Free PMC article.
-
Osteoblastic induction on calcium phosphate cement-chitosan constructs for bone tissue engineering.J Biomed Mater Res A. 2010 Jul;94(1):223-33. doi: 10.1002/jbm.a.32665. J Biomed Mater Res A. 2010. PMID: 20166217 Free PMC article.
-
Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering.Dent Mater. 2019 Jul;35(7):1031-1041. doi: 10.1016/j.dental.2019.04.009. Epub 2019 May 7. Dent Mater. 2019. PMID: 31076156 Review.
-
Stem Cells and Calcium Phosphate Cement Scaffolds for Bone Regeneration.J Dent Res. 2014 Jul;93(7):618-25. doi: 10.1177/0022034514534689. Epub 2014 May 5. J Dent Res. 2014. PMID: 24799422 Free PMC article. Review.
Cited by
-
Three-Dimensional Impression of Biomaterials for Alveolar Graft: Scoping Review.J Funct Biomater. 2023 Jan 29;14(2):76. doi: 10.3390/jfb14020076. J Funct Biomater. 2023. PMID: 36826875 Free PMC article.
-
Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair.Acta Biomater. 2010 Oct;6(10):4118-26. doi: 10.1016/j.actbio.2010.04.029. Epub 2010 May 6. Acta Biomater. 2010. PMID: 20451676 Free PMC article.
-
Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration.Acta Biomater. 2012 Jul;8(6):2297-306. doi: 10.1016/j.actbio.2012.02.021. Epub 2012 Mar 3. Acta Biomater. 2012. PMID: 22391411 Free PMC article.
-
Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments.J Orthop Translat. 2014 Dec 12;3(1):1-11. doi: 10.1016/j.jot.2014.11.002. eCollection 2015 Jan. J Orthop Translat. 2014. PMID: 30035034 Free PMC article. Review.
-
Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration.Tissue Eng Part A. 2014 Apr;20(7-8):1295-305. doi: 10.1089/ten.TEA.2013.0211. Epub 2014 Jan 7. Tissue Eng Part A. 2014. PMID: 24279868 Free PMC article.
References
-
- Nguyen PN, Sullivan PK. Issues and controversies in the management of cleft palate. Clin Plast Surg. 1993;20:671–682. - PubMed
-
- Phillips JH, Forrest CR, Gruss JS. Current concepts in the use of bone grafts in facial fractures. Basic science considerations. Clin Plast Surg. 1992;19:41–58. - PubMed
-
- Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4:743–765. - PubMed
-
- Laurencin CT, Ambrosio AM, Borden MD, Cooper JA., Jr Tissue engineering: Orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. - PubMed
-
- Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res. 1999;367:S95–S106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

