Alpha4 is a ubiquitin-binding protein that regulates protein serine/threonine phosphatase 2A ubiquitination
- PMID: 20092282
- PMCID: PMC2832797
- DOI: 10.1021/bi901837h
Alpha4 is a ubiquitin-binding protein that regulates protein serine/threonine phosphatase 2A ubiquitination
Abstract
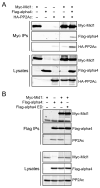
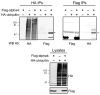
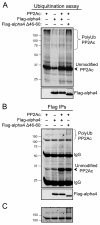
Multiple regulatory mechanisms control the activity of the protein serine/threonine phosphatase 2A catalytic subunit (PP2Ac), including post-translational modifications and its association with regulatory subunits and interacting proteins. Alpha4 is a PP2Ac-interacting protein that is hypothesized to play a role in PP2Ac ubiquitination via its interaction with the E3 ubiquitin ligase Mid1. In this report, we show that alpha4 serves as a necessary adaptor protein that provides a binding platform for both PP2Ac and Mid1. We also identify a novel ubiquitin-interacting motif (UIM) within alpha4 (amino acid residues 46-60) and analyze the interaction between alpha4 and ubiquitin using NMR. Consistent with other UIM-containing proteins, alpha4 is monoubiquitinated. Interestingly, deletion of the UIM within alpha4 enhances its association with polyubiquitinated proteins. Lastly, we demonstrate that addition of wild-type alpha4 but not an alpha4 UIM deletion mutant suppresses PP2Ac polyubiquitination. Thus, the polyubiquitination of PP2Ac is inhibited by the UIM within alpha4. These findings reveal direct regulation of PP2Ac polyubiquitination by a novel UIM within the adaptor protein alpha4.
Figures


Similar articles
-
MID1 catalyzes the ubiquitination of protein phosphatase 2A and mutations within its Bbox1 domain disrupt polyubiquitination of alpha4 but not of PP2Ac.PLoS One. 2014 Sep 10;9(9):e107428. doi: 10.1371/journal.pone.0107428. eCollection 2014. PLoS One. 2014. PMID: 25207814 Free PMC article.
-
The E3 ubiquitin ligase- and protein phosphatase 2A (PP2A)-binding domains of the Alpha4 protein are both required for Alpha4 to inhibit PP2A degradation.J Biol Chem. 2011 May 20;286(20):17665-71. doi: 10.1074/jbc.M111.222414. Epub 2011 Mar 29. J Biol Chem. 2011. PMID: 21454489 Free PMC article.
-
The MID1 E3 ligase catalyzes the polyubiquitination of Alpha4 (α4), a regulatory subunit of protein phosphatase 2A (PP2A): novel insights into MID1-mediated regulation of PP2A.J Biol Chem. 2013 Jul 19;288(29):21341-21350. doi: 10.1074/jbc.M113.481093. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740247 Free PMC article.
-
The MID1 gene product in physiology and disease.Gene. 2020 Jul 15;747:144655. doi: 10.1016/j.gene.2020.144655. Epub 2020 Apr 10. Gene. 2020. PMID: 32283114 Free PMC article. Review.
-
Structural Diversity of Ubiquitin E3 Ligase.Molecules. 2021 Nov 4;26(21):6682. doi: 10.3390/molecules26216682. Molecules. 2021. PMID: 34771091 Free PMC article. Review.
Cited by
-
Functional characterization of chaperonin containing T-complex polypeptide-1 and its conserved and novel substrates in Arabidopsis.J Exp Bot. 2019 May 9;70(10):2741-2757. doi: 10.1093/jxb/erz099. J Exp Bot. 2019. PMID: 30825377 Free PMC article.
-
Protein phosphatase 2A - structure, function and role in neurodevelopmental disorders.J Cell Sci. 2021 Jul 1;134(13):jcs248187. doi: 10.1242/jcs.248187. Epub 2021 Jul 6. J Cell Sci. 2021. PMID: 34228795 Free PMC article. Review.
-
Multiple E3 ligases control tankyrase stability and function.Nat Commun. 2023 Nov 8;14(1):7208. doi: 10.1038/s41467-023-42939-3. Nat Commun. 2023. PMID: 37938264 Free PMC article.
-
The ubiquitin E3 ligase NOSIP modulates protein phosphatase 2A activity in craniofacial development.PLoS One. 2014 Dec 29;9(12):e116150. doi: 10.1371/journal.pone.0116150. eCollection 2014. PLoS One. 2014. PMID: 25546391 Free PMC article.
-
The three Type 2A protein phosphatases, PP2Ac, PP4c and PP6c, are differentially regulated by Alpha4.Biochem Biophys Res Commun. 2016 Jun 17;475(1):64-9. doi: 10.1016/j.bbrc.2016.05.036. Epub 2016 May 8. Biochem Biophys Res Commun. 2016. PMID: 27169767 Free PMC article.
References
-
- Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. - PubMed
-
- Trockenbacher A, Suckow V, Foerster J, Winter J, Krauss S, Ropers HH, Schneider R, Schweiger S. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29:287–294. - PubMed
-
- Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. - PubMed
-
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK070787/DK/NIDDK NIH HHS/United States
- T32 CA009582/CA/NCI NIH HHS/United States
- MH19732/MH/NIMH NIH HHS/United States
- GM051366/GM/NIGMS NIH HHS/United States
- T32 MH019732/MH/NIMH NIH HHS/United States
- T32 CA09582/CA/NCI NIH HHS/United States
- R01 GM051366/GM/NIGMS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 GM075156/GM/NIGMS NIH HHS/United States
- R56 GM051366/GM/NIGMS NIH HHS/United States
- DK070787/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- GM075156/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

