Protein modification, bioconjugation, and disulfide bridging using bromomaleimides
- PMID: 20092331
- PMCID: PMC2842020
- DOI: 10.1021/ja908610s
Protein modification, bioconjugation, and disulfide bridging using bromomaleimides
Abstract
The maleimide motif is widely used for the selective chemical modification of cysteine residues in proteins. Despite widespread utilization, there are some potential limitations, including the irreversible nature of the reaction and, hence, the modification and the number of attachment positions. We conceived of a new class of maleimide which would address some of these limitations and provide new opportunities for protein modification. We report herein the use of mono- and dibromomaleimides for reversible cysteine modification and illustrate this on the SH2 domain of the Grb2 adaptor protein (L111C). After initial modification of a protein with a bromo- or dibromomaleimide, it is possible to add an equivalent of a second thiol to give further bioconjugation, demonstrating that bromomaleimides offer opportunities for up to three points of attachment. The resultant protein-maleimide products can be cleaved to regenerate the unmodified protein by addition of a phosphine or a large excess of a thiol. Furthermore, dibromomaleimide can insert into a disulfide bond, forming a maleimide bridge, and this is illustrated on the peptide hormone somatostatin. Fluorescein-labeled dibromomaleimide is synthesized and inserted into the disulfide to construct a fluorescent somatostatin analogue. These results highlight the significant potential for this new class of reagents in protein modification.
Figures
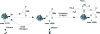
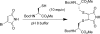

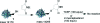

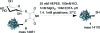

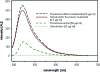
References
-
- Lundblad R. L.Chemical Reagents for Protein Modification, 3rd ed.; CRC Press: Boca Raton, FL, 2005.
-
- Chalker J. M.; Bernardes G. J. L.; Lin Y. A.; Davis B. G. Chem. Asian J. 2009, 4, 630–640. - PubMed
-
- Fodje M. N.; Al-Karadaghi S. Protein Eng. 2002, 15, 353–358. - PubMed
-
- Hermanson G. T.Bioconjugate Techniques; Academic Press: London, 1996.
-
- Schelte P.; Boeckler C.; Frisch B.; Schuber F. Bioconjugate Chem. 2000, 11, 118–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

