Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells
- PMID: 20092614
- PMCID: PMC2845099
- DOI: 10.1186/1741-7007-8-6
Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells
Abstract
Background: Folate (vitamin B9) is essential for cellular proliferation as it is involved in the biosynthesis of deoxythymidine monophosphate (dTMP) and s-adenosylmethionine (AdoMet). The link between folate depletion and the genesis and progression of cancers of epithelial origin is of high clinical relevance, but still unclear. We recently demonstrated that sensitivity to low folate availability is affected by the rate of polyamine biosynthesis, which is prominent in prostate cells. We, therefore, hypothesized that prostate cells might be highly susceptible to genetic, epigenetic and phenotypic changes consequent to folate restriction.
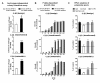
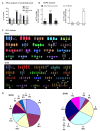
Results: We studied the consequences of long-term, mild folate depletion in a model comprised of three syngenic cell lines derived from the transgenic adenoma of the mouse prostate (TRAMP) model, recapitulating different stages of prostate cancer; benign, transformed and metastatic. High-performance liquid chromatography analysis demonstrated that mild folate depletion (100 nM) sufficed to induce imbalance in both the nucleotide and AdoMet pools in all prostate cell lines. Random oligonucleotide-primed synthesis (ROPS) revealed a significant increase in uracil misincorporation and DNA single strand breaks, while spectral karyotype analysis (SKY) identified five novel chromosomal rearrangements in cells grown with mild folate depletion. Using global approaches, we identified an increase in CpG island and histone methylation upon folate depletion despite unchanged levels of total 5-methylcytosine, indicating a broad effect of folate depletion on epigenetic regulation. These genomic changes coincided with phenotype changes in the prostate cells including increased anchorage-independent growth and reduced sensitivity to folate depletion.
Conclusions: This study demonstrates that prostate cells are highly susceptible to genetic and epigenetic changes consequent to mild folate depletion as compared to cells grown with supraphysiological amounts of folate (2 microM) routinely used in tissue culture. In addition, we elucidate for the first time the contribution of these aspects to consequent phenotype changes in epithelial cells. These results provide a strong rationale for studying the effects of folate manipulation on the prostate in vivo, where cells might be more sensitive to changes in folate status resulting from folate supplementation or antifolate therapeutic approaches.
Figures


Similar articles
-
Dietary folate deficiency blocks prostate cancer progression in the TRAMP model.Cancer Prev Res (Phila). 2011 Nov;4(11):1825-34. doi: 10.1158/1940-6207.CAPR-11-0140. Epub 2011 Aug 11. Cancer Prev Res (Phila). 2011. PMID: 21836022 Free PMC article.
-
Polyamine biosynthesis impacts cellular folate requirements necessary to maintain S-adenosylmethionine and nucleotide pools.FASEB J. 2009 Sep;23(9):2888-97. doi: 10.1096/fj.09-130708. Epub 2009 May 5. FASEB J. 2009. PMID: 19417083 Free PMC article.
-
Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells.Nutr Cancer. 2000;37(2):245-51. doi: 10.1207/S15327914NC372_18. Nutr Cancer. 2000. PMID: 11142099
-
Beyond the island: epigenetic biomarkers of colorectal and prostate cancer.Methods Mol Biol. 2015;1238:103-24. doi: 10.1007/978-1-4939-1804-1_6. Methods Mol Biol. 2015. PMID: 25421657 Review.
-
Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity.Mutat Res. 2012 May 1;733(1-2):21-33. doi: 10.1016/j.mrfmmm.2011.11.003. Epub 2011 Nov 7. Mutat Res. 2012. PMID: 22093367 Review.
Cited by
-
From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition?Clin Epigenetics. 2015 Mar 25;7(1):33. doi: 10.1186/s13148-015-0068-2. eCollection 2015. Clin Epigenetics. 2015. PMID: 25861393 Free PMC article.
-
Effects of administration of co-trimoxazole and folic acid on sperm quality and histological changes of testes in male rats.Int J Reprod Biomed. 2017 Oct;15(10):625-634. Int J Reprod Biomed. 2017. PMID: 29387828 Free PMC article.
-
Pharmacological polyamine catabolism upregulation with methionine salvage pathway inhibition as an effective prostate cancer therapy.Nat Commun. 2020 Jan 7;11(1):52. doi: 10.1038/s41467-019-13950-4. Nat Commun. 2020. PMID: 31911608 Free PMC article.
-
Impact of folate intake on prostate cancer recurrence following definitive therapy: data from CaPSURE™.J Urol. 2014 Apr;191(4):971-6. doi: 10.1016/j.juro.2013.09.065. Epub 2013 Oct 3. J Urol. 2014. PMID: 24095905 Free PMC article.
-
Decreased autophagy was implicated in the decreased apoptosis during decidualization in early pregnant mice.J Mol Histol. 2018 Dec;49(6):589-597. doi: 10.1007/s10735-018-9797-9. Epub 2018 Oct 8. J Mol Histol. 2018. PMID: 30298448
References
-
- Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. - PubMed
-
- Pogribny IP, James SJ, Jernigan S, Pogribna M. Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat Res. 2004;548:53–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

