Assessment of two malaria rapid diagnostic tests in children under five years of age, with follow-up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone
- PMID: 20092620
- PMCID: PMC2835716
- DOI: 10.1186/1475-2875-9-28
Assessment of two malaria rapid diagnostic tests in children under five years of age, with follow-up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone
Abstract
Background: Most malaria rapid diagnostic tests (RDTs) use HRP2 detection, including Paracheck-Pf(R), but their utility is limited by persistent false positivity after treatment. PLDH-based tests become negative more quickly, but sensitivity has been reported below the recommended standard of 90%. A new pLDH test, CareStart three-line P.f/PAN-pLDH, claims better sensitivity with continued rapid conversion to negative. The study aims were to 1) compare sensitivity and specificity of CareStart to Paracheck-Pf(R) to diagnose falciparum malaria in children under five years of age, 2) assess how quickly false-positive CareStart tests become negative and 3) evaluate ease of use and inter-reader agreement of both tests.
Methods: Participants were included if they were aged between two and 59 months, presenting to a Médecins Sans Frontières community health centre in eastern Sierra Leone with suspected malaria defined as fever (axillary temperature > 37.5 degrees C) and/or history of fever in the previous 72 hours and no signs of severe disease. The same capillary blood was used for the RDTs and the blood slide, the latter used as the gold standard reference. All positive participants were treated with supervised artesunate and amodiaquine treatment for three days. Participants with a persistent false-positive CareStart, but a negative blood slide on Day 2, were followed with repeated CareStart and blood slide tests every seven days until CareStart became negative or a maximum of 28 days.
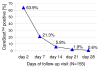
Results: Sensitivity of CareStart was 99.4% (CI 96.8-100.0, 168/169) and of Paracheck-Pf(R), 98.8% (95% CI 95.8-99.8, 167/169). Specificity of CareStart was 96.0% (CI 91.9-98.4, 167/174) and of Paracheck-Pf(R), 74.7% (CI 67.6-81.0, 130/174) (p < 0.001). Neither test showed any change in sensitivity with decreasing parasitaemia. Of the 155 eligible follow-up CareStart participants, 63.9% (99/155) had a false-positive test on day 2, 21.3% (33/155) on day 7, 5.8% (9/155) on day 14, 1.9% (3/155) on day 21 and 0.6% (1/155) on day 28. The median time for test negativity was seven days. CareStart was as easy to use and interpret as Paracheck-Pf(R) with excellent inter-reader agreement.
Conclusions: Both RDTs were highly sensitive, met WHO standards for the detection of falciparum malaria monoinfections where parasitaemia was >100 parasites/mul and were easy to use. CareStart persistent false positivity decreased quickly after successful anti-malarial treatment, making it a good choice for a RDT for a hyperendemic falciparum malaria area.
Figures
References
-
- World Health Organization. World malaria report, 2008. Geneva; 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous