Analysis of HmsH and its role in plague biofilm formation
- PMID: 20093287
- PMCID: PMC2889448
- DOI: 10.1099/mic.0.036640-0
Analysis of HmsH and its role in plague biofilm formation
Abstract
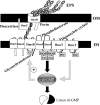
The Yersinia pestis Hms(+) phenotype is a manifestation of biofilm formation that causes adsorption of Congo red and haemin at 26 degrees C but not at 37 degrees C. This phenotype is required for blockage of the proventricular valve of the oriental rat flea and plays a role in transmission of bubonic plague from fleas to mammals. Genes responsible for this phenotype are located in three separate operons, hmsHFRS, hmsT and hmsP. HmsH and HmsF are outer membrane (OM) proteins, while the other four Hms proteins are located in the inner membrane. According to the Hidden Markov Method-based predictor, HmsH has a large N terminus in the periplasm, a beta-barrel structure with 16 beta-strands that traverse the OM, eight surface-exposed loops, and seven short turns connecting the beta-strands on the periplasmic side. Here, we demonstrate that HmsH is a heat-modifiable protein, a characteristic of other beta-barrel proteins, thereby supporting the bioinformatics analysis. Alanine scanning mutagenesis was used to identify conserved amino acids in the HmsH-like family that are critical for the function of HmsH in biofilm formation. Of 23 conserved amino acids mutated, four residues affected HmsH function and three likely caused protein instability. We used formaldehyde cross-linking to demonstrate that HmsH interacts with HmsF but not with HmsR, HmsS, HmsT or HmsP. Loss-of-function HmsH variants with single alanine substitutions retained their beta-structure and interaction with HmsF. Finally, using a polar hmsH : : mini-kan mutant, we demonstrated that biofilm development is not important for the pathogenesis of bubonic or pneumonic plague in mice.
Figures




References
-
- Ausubel, F. M. (1987). Current protocols in Molecular Biology. New York: Greene Publishing & Wiley-Interscience.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

