Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients
- PMID: 20093343
- PMCID: PMC2849684
- DOI: 10.2215/CJN.07371009
Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients
Abstract
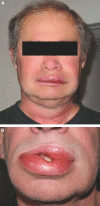
Background and objective: The clinical manifestation of angioedema ranges from minor facial edema up to life-threatening swelling of mouth and throat. Hereditary defects, drugs, and food allergies may play a role in the development of angioedema. We systematically investigated the incidence of angioedema in renal allograft recipients treated with mTOR inhibitors (mTORis).
Design, setting, participants, & measurements: All patients in the authors' electronic database who had received mTORis (n = 309) between 2000 and 2008 were identified. Of these, 137 were additionally treated with angiotensin-converting enzyme inhibitors (ACEis).
Results: Nine patients (6.6%, 3.8 per 100 treatment years) developed angioedema after a mean period of 123 days under combined therapy with mTORi and ACEi. Among the remaining 172 patients on mTORi, including 119 patients treated with angiotensin-receptor blockers, only two developed angioedema (1.2%, 0.5 per 100 treatment years, P = 0.01). In patients receiving mycophenolate and ACEi (n = 462), 10 instances of angioedema were found (2.1%, 0.8 per 100 treatment years, P = 0.004).
Conclusions: This systematic investigation demonstrated a noticeable incidence of 6.6% angioedema under combined therapy with mTORi and ACEi in kidney transplant recipients. Treatment with either ACEi or mTORi alone resulted in a significantly lower incidence of angioedema, suggesting that this combination should be avoided.
Figures
References
-
- Donaldson VH, Evans RR: A biochemical abnormality in herediatry angioneurotic edema: Absence of serum inhibitor of C' 1-esterase. Am J Med 35: 37–44, 1963 - PubMed
-
- Zuraw BL: Clinical practice. Hereditary angioedema. N Engl J Med 359: 1027–1036, 2008 - PubMed
-
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G: Nonallergic angioedema: Role of bradykinin. Allergy 62: 842–856, 2007 - PubMed
-
- Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ: ACE inhibitor-induced angioedema. Incidence, prevention and management. Drug Saf 18: 171–188, 1998 - PubMed
-
- Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E: Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 165: 1637–1642, 2005 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous