A new type of signal peptidase cleavage site identified in an RNA virus polyprotein
- PMID: 20093364
- PMCID: PMC2838279
- DOI: 10.1074/jbc.M109.083394
A new type of signal peptidase cleavage site identified in an RNA virus polyprotein
Abstract
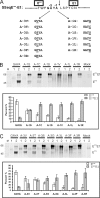
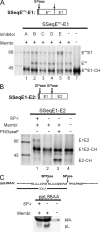
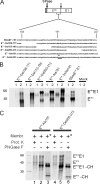
Pestiviruses, a group of enveloped positive strand RNA viruses belonging to the family Flaviviridae, express their genes via a polyprotein that is subsequently processed by proteases. The structural protein region contains typical signal peptidase cleavage sites. Only the site at the C terminus of the glycoprotein E(rns) is different because it does not contain a hydrophobic transmembrane region but an amphipathic helix functioning as the E(rns) membrane anchor. Despite the absence of a hydrophobic region, the site between the C terminus of E(rns) and E1, the protein located downstream in the polyprotein, is cleaved by signal peptidase, as demonstrated by mutagenesis and inhibitor studies. Thus, E(rns)E1 is processed at a novel type of signal peptidase cleavage site showing a different membrane topology. Prevention of glycosylation or introduction of mutations into the C-terminal region of E(rns) severely impairs processing, presumably by preventing proper membrane interaction or disturbing a conformation critical for the protein to be accepted as a substrate by signal peptidase.
Figures





References
-
- Heinz F. X., Collett M. S., Purcell R. H., Gould E. A., Howard C. R., Houghton M., Moormann J. M., Rice C. M., Thiel H. J. (2000) in Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses (van Regenmortel M. H. V., Fauquet C. M., Bishop D. H. L., Carstens E. B., Estes M. K., Lemon S. M., Maniloff J., Mayo M. A., McGeoch D. J., Pringle C. R., Wickner R. B. eds) pp. 859–878, Academic Press, Inc., San Diego, CA
-
- Lindenbach B. D., Thiel H.-J., Rice C. M. (2007) in Fields Virology (Knipe D. M., Howley P. M. eds) pp. 1101–1152, Lippincott-Raven Publishers, Philadelphia
-
- Collett M. S., Larson R., Belzer S. K., Retzel E. (1988) Virology 165, 200–208 - PubMed
-
- Collett M. S., Moennig V., Horzinek M. C. (1989) J. Gen. Virol. 70, 253–266 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

