Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival
- PMID: 20093365
- PMCID: PMC2843224
- DOI: 10.1074/jbc.M109.043117
Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival
Abstract
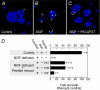
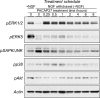
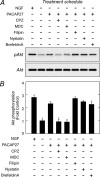
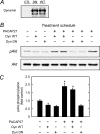
MAPK and Akt pathways are predominant mediators of trophic signaling for many neuronal systems. Among the vasoactive intestinal peptide/secretin/glucagon family of related peptides, pituitary adenylate cyclase-activating polypeptide (PACAP) binding to specific PAC(1) receptor isoforms can engage multiple signaling pathways and promote neuroprotection through mechanisms that are not well understood. Using a primary sympathetic neuronal system, the current studies demonstrate that PACAP activation of PAC(1)HOP1 receptors engages both MAPK and Akt neurotrophic pathways in an integrated program to facilitate neuronal survival after growth factor withdrawal. PACAP not only stimulated prosurvival ERK1/2 and ERK5 activation but also abrogated SAPK/JNK and p38 MAPK signaling in parallel. In contrast to the potent and rapid effects of PACAP in ERK1/2 phosphorylation, PACAP stimulated Akt phosphorylation in a late phase of PAC(1)HOP1 receptor signaling. From inhibitor and immunoprecipitation analyses, the PACAP/PAC(1)HOP1 receptor-mediated Akt responses did not represent transactivation mechanisms but appeared to depend on G alpha(q)/phosphatidylinositol 3-kinase gamma activity and vesicular internalization pathways. Phosphatidylinositol 3-kinase gamma-selective inhibitors blocked PACAP-stimulated Akt phosphorylation in primary neuronal cultures and in PAC(1)HOP1-overexpressing cell lines; RNA interference-mediated knockdown of the receptor effectors attenuated PACAP-mediated Akt activation. Similarly, perturbation of endocytic pathways also blocked Akt phosphorylation. Between ERK and Akt pathways, PACAP-stimulated Akt signaling was the primary cascade that attenuated cultured neuron apoptosis after growth factor withdrawal. The partitioning of PACAP-mediated Akt signaling in endosomes may be a key mechanism contributing to the high spatial and temporal specificity in signal transduction necessary for survival pathways.
Figures




Similar articles
-
PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability.J Mol Neurosci. 2019 Jul;68(3):340-347. doi: 10.1007/s12031-018-1127-x. Epub 2018 Jul 27. J Mol Neurosci. 2019. PMID: 30054797 Free PMC article. Review.
-
PAC1 Receptor Internalization and Endosomal MEK/ERK Activation Is Essential for PACAP-Mediated Neuronal Excitability.J Mol Neurosci. 2021 Aug;71(8):1536-1542. doi: 10.1007/s12031-021-01821-x. Epub 2021 Mar 6. J Mol Neurosci. 2021. PMID: 33675454 Free PMC article. Review.
-
Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability.J Neurosci. 2013 Mar 6;33(10):4614-22. doi: 10.1523/JNEUROSCI.4999-12.2013. J Neurosci. 2013. PMID: 23467377 Free PMC article.
-
Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways.J Biol Chem. 1999 Sep 24;274(39):27702-10. doi: 10.1074/jbc.274.39.27702. J Biol Chem. 1999. PMID: 10488112
-
Signaling pathways activated by PACAP in MCF-7 breast cancer cells.Cell Signal. 2018 Oct;50:37-47. doi: 10.1016/j.cellsig.2018.06.009. Epub 2018 Jun 20. Cell Signal. 2018. PMID: 29935235
Cited by
-
Secretin receptor promotes the proliferation of endocrine tumor cells via the PI3K/AKT pathway.Mol Endocrinol. 2012 Aug;26(8):1394-405. doi: 10.1210/me.2012-1055. Epub 2012 Jun 12. Mol Endocrinol. 2012. PMID: 22692904 Free PMC article.
-
PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability.J Mol Neurosci. 2019 Jul;68(3):340-347. doi: 10.1007/s12031-018-1127-x. Epub 2018 Jul 27. J Mol Neurosci. 2019. PMID: 30054797 Free PMC article. Review.
-
Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis.Am J Physiol Endocrinol Metab. 2022 Mar 1;322(3):E199-E210. doi: 10.1152/ajpendo.00320.2021. Epub 2022 Jan 10. Am J Physiol Endocrinol Metab. 2022. PMID: 35001657 Free PMC article.
-
PAC1 Receptor Internalization and Endosomal MEK/ERK Activation Is Essential for PACAP-Mediated Neuronal Excitability.J Mol Neurosci. 2021 Aug;71(8):1536-1542. doi: 10.1007/s12031-021-01821-x. Epub 2021 Mar 6. J Mol Neurosci. 2021. PMID: 33675454 Free PMC article. Review.
-
Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability.J Neurosci. 2013 Mar 6;33(10):4614-22. doi: 10.1523/JNEUROSCI.4999-12.2013. J Neurosci. 2013. PMID: 23467377 Free PMC article.
References
-
- Sherwood N. M., Krueckl S. L., McRory J. E. (2000) Endocr. Rev. 21, 619–670 - PubMed
-
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. (1993) Nature 365, 170–175 - PubMed
-
- Lutz E. M., Sheward W. J., West K. M., Morrow J. A., Fink G., Harmar A. J. (1993) FEBS Lett. 334, 3–8 - PubMed
-
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. (1992) Neuron. 8, 811–819 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

