Energy restriction as an antitumor target of thiazolidinediones
- PMID: 20093366
- PMCID: PMC2843227
- DOI: 10.1074/jbc.M109.065466
Energy restriction as an antitumor target of thiazolidinediones
Abstract
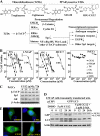
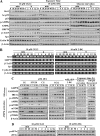
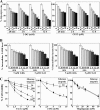
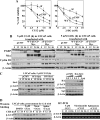
Cancer cells gain growth advantages in the microenvironment by shifting cellular metabolism to aerobic glycolysis, the so-called Warburg effect. There is a growing interest in targeting aerobic glycolysis for cancer therapy by exploiting the differential susceptibility of malignant versus normal cells to glycolytic inhibition, of which the proof-of-concept is provided by the in vivo efficacy of dietary caloric restriction and natural product-based energy restriction-mimetic agents (ERMAs) such as resveratrol and 2-deoxyglucose in suppressing carcinogenesis in animal models. Here, we identified thiazolidinediones as a novel class of ERMAs in that they elicited hallmark cellular responses characteristic of energy restriction, including transient induction of Sirt1 (silent information regulator 1) expression, activation of the intracellular fuel sensor AMP-activated protein kinase, and endoplasmic reticulum stress, the interplay among which culminated in autophagic and apoptotic death. The translational implications of this finding are multifold. First, the novel function of troglitazone and ciglitazone in targeting energy restriction provides a mechanistic basis to account for their peroxisome proliferator-activated receptor gamma-independent effects on a broad spectrum of signaling targets. Second, we demonstrated that Sirt1-mediated up-regulation of beta-transducin repeat-containing protein-facilitated proteolysis of cell cycle- and apoptosis-regulatory proteins is an energy restriction-elicited signaling event and is critical for the antitumor effects of ERMAs. Third, it provides a molecular rationale for using thiazolidinediones as scaffolds to develop potent ERMAs, of which the proof-of-principle is demonstrated by OSU-CG12. OSU-CG12, a peroxisome proliferator-activated receptor gamma-inactive ciglitazone derivative, exhibits 1- and 3-order of magnitude higher potency in eliciting starvation-like cellular responses relative to resveratrol and 2-deoxyglucose, respectively.
Figures




References
-
- Yki-Järvinen H. (2004) N. Engl. J. Med. 351, 1106–1118 - PubMed
-
- Panchapakesan U., Chen X. M., Pollock C. A. (2005) Nat. Clin. Pract. Nephrol. 1, 33–43 - PubMed
-
- Sharma A. M., Staels B. (2007) J. Clin. Endocrinol. Metab. 92, 386–395 - PubMed
-
- Grommes C., Landreth G. E., Heneka M. T. (2004) Lancet Oncol. 5, 419–429 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

