Gated access to the pore of a P2X receptor: structural implications for closed-open transitions
- PMID: 20093367
- PMCID: PMC2843173
- DOI: 10.1074/jbc.M109.089185
Gated access to the pore of a P2X receptor: structural implications for closed-open transitions
Abstract
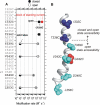
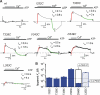
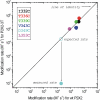
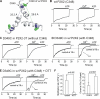
P2X receptors are ligand-gated cation channels that transition from closed to open states upon binding ATP. The crystal structure of the closed zebrafish P2X4.1 receptor directly reveals that the ion-conducting pathway is formed by three transmembrane domain 2 (TM2) alpha-helices, each being provided by the three subunits of the trimer. However, the transitions in TM2 that accompany channel opening are incompletely understood and remain unresolved. In this study, we quantified gated access to Cd(2+) at substituted cysteines in TM2 of P2X2 receptors in the open and closed states. Our data for the closed state are consistent with the zebrafish P2X4.1 structure, with isoleucines and threonines (Ile-332 and Thr-336) positioned one helical turn apart lining the channel wall on approach to the gate. Our data for the open state reveal gated access to deeper parts of the pore (Thr-339, Val-343, Asp-349, and Leu-353), suggesting the closed channel gate is between Thr-336 and Thr-339. We also found unexpected interactions between native Cys-348 and D349C that result in tight Cd(2+) binding deep within the intracellular vestibule in the open state. Interpreted with a P2X2 receptor structural model of the closed state, our data suggest that the channel gate opens near Thr-336/Thr-339 and is accompanied by movement of the pore-lining regions, which narrow toward the cytosolic end of TM2 in the open state. Such transitions would relieve the barrier to ion flow and render the intracellular vestibule less splayed during channel opening in the presence of ATP.
Figures



Similar articles
-
Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification.J Neurosci. 2009 Nov 11;29(45):14257-64. doi: 10.1523/JNEUROSCI.4403-09.2009. J Neurosci. 2009. PMID: 19906973 Free PMC article.
-
Crystal structure of the ATP-gated P2X(4) ion channel in the closed state.Nature. 2009 Jul 30;460(7255):592-8. doi: 10.1038/nature08198. Nature. 2009. PMID: 19641588 Free PMC article.
-
Direct gating of ATP-activated ion channels (P2X2 receptors) by lipophilic attachment at the outer end of the second transmembrane domain.J Biol Chem. 2014 Jan 10;289(2):618-26. doi: 10.1074/jbc.M113.529099. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273165 Free PMC article.
-
Bridging the gap between structural bioinformatics and receptor research: the membrane-embedded, ligand-gated, P2X glycoprotein receptor.Curr Top Med Chem. 2004;4(16):1657-705. doi: 10.2174/1568026043387197. Curr Top Med Chem. 2004. PMID: 15579102 Review.
-
Molecular properties of P2X receptors.Pflugers Arch. 2006 Aug;452(5):486-500. doi: 10.1007/s00424-006-0073-6. Epub 2006 Apr 11. Pflugers Arch. 2006. PMID: 16607539 Review.
Cited by
-
Roles of the lateral fenestration residues of the P2X₄ receptor that contribute to the channel function and the deactivation effect of ivermectin.Purinergic Signal. 2015 Jun;11(2):229-38. doi: 10.1007/s11302-015-9448-5. Epub 2015 Apr 7. Purinergic Signal. 2015. PMID: 25847072 Free PMC article.
-
Size matters in activation/inhibition of ligand-gated ion channels.Trends Pharmacol Sci. 2012 Sep;33(9):482-93. doi: 10.1016/j.tips.2012.06.005. Epub 2012 Jul 11. Trends Pharmacol Sci. 2012. PMID: 22789930 Free PMC article. Review.
-
Post-translational regulation of P2X receptor channels: modulation by phospholipids.Front Cell Neurosci. 2013 Nov 25;7:226. doi: 10.3389/fncel.2013.00226. Front Cell Neurosci. 2013. PMID: 24324400 Free PMC article. Review.
-
Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells.Cell Mol Neurobiol. 2010 Nov;30(8):1251-8. doi: 10.1007/s10571-010-9568-y. Epub 2010 Nov 25. Cell Mol Neurobiol. 2010. PMID: 21107680 Free PMC article. Review.
-
Multiple roles of the extracellular vestibule amino acid residues in the function of the rat P2X4 receptor.PLoS One. 2013;8(3):e59411. doi: 10.1371/journal.pone.0059411. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555667 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

