Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates
- PMID: 20093376
- PMCID: PMC2855384
- DOI: 10.1099/jmm.0.017715-0
Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates
Abstract
Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen capable of acutely infecting or persistently colonizing susceptible hosts. P. aeruginosa colonizes surfaces in vitro by either biofilm formation or swarming motility. The choice of behaviour is influenced by the physical properties of the surface and specific nutrient availability, and subject to regulatory networks that also govern type 2 and type 3 protein secretion. Biofilm formation by clinical isolates has been well-studied. However, the swarming behaviour of human isolates has not been extensively analysed. We collected isolates from 237 hospitalized patients without cystic fibrosis and analysed motility and secretion phenotypes of each isolate. We found biofilm formation and swarming to be negatively associated, while swarming was positively associated with the secretion of both proteases and type 3 exoenzymes. Most isolates were capable of type 3 secretion and biofilm formation, even though these traits are considered to favour distinct modes of pathogenesis. Our data demonstrate that while clinical isolates display diverse motility, biofilm and secretion phenotypes, many of the predicted relationships between swarming motility and other phenotypes observed in laboratory strains also hold true for bacteria isolated from human patients.
Figures
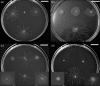

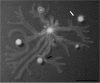
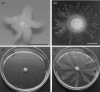

References
-
- Balloy, V., Verma, A., Kuravi, S., Si-Tahar, M., Chignard, M. & Ramphal, R. (2007). The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis 196, 289–296. - PubMed
-
- Boles, B. R., Thoendel, M. & Singh, P. K. (2005). Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57, 1210–1223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

