Repulsion of superinfecting virions: a mechanism for rapid virus spread
- PMID: 20093437
- PMCID: PMC4202693
- DOI: 10.1126/science.1183173
Repulsion of superinfecting virions: a mechanism for rapid virus spread
Abstract
Viruses are thought to spread across susceptible cells through an iterative process of infection, replication, and release, so that the rate of spread is limited by replication kinetics. Here, we show that vaccinia virus spreads across one cell every 75 minutes, fourfold faster than its replication cycle would permit. To explain this phenomenon, we found that newly infected cells express two surface proteins that mark cells as infected and, via exploitation of cellular machinery, induce the repulsion of superinfecting virions away toward uninfected cells. Mechanistically, early expression of proteins A33 and A36 was critical for virion repulsion and rapid spread, and cells expressing these proteins repelled exogenous virions rapidly. Additional spreading mechanisms may exist for other viruses that also spread faster than predicted by replication kinetics.
Figures
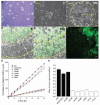


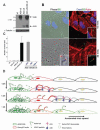
Comment in
-
Cell biology. Propelling progeny.Science. 2010 Feb 12;327(5967):787-8. doi: 10.1126/science.1187160. Science. 2010. PMID: 20150470 No abstract available.
References
-
- Moss B. In: Fields Virology. Knipe DM, editor. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2905–2946.
-
- Condit RC, Moussatche N, Traktman P. Adv. Virus Res. 2006;66:31. - PubMed
-
- Smith GL, Vanderplasschen A, Law M. J. Gen. Virol. 2002;83:2915. - PubMed
-
- Roberts KL, Smith GL. Trends Microbiol. 2008;16:472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

