Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis
- PMID: 20093491
- PMCID: PMC2830821
- DOI: 10.2353/ajpath.2010.090503
Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis
Abstract
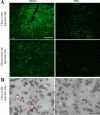
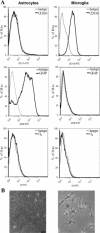
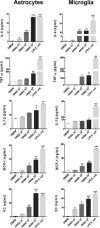
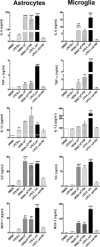
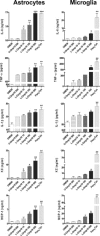
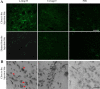
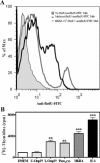
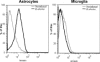
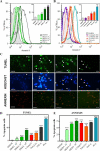
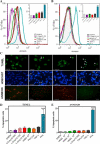
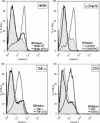
Central nervous system (CNS) invasion by bacteria of the genus Brucella results in an inflammatory disorder called neurobrucellosis. In this study we present in vivo and in vitro evidence that B. abortus and its lipoproteins activate the innate immunity of the CNS, eliciting an inflammatory response that leads to astrogliosis, a characteristic feature of neurobrucellosis. Intracranial injection of heat-killed B. abortus (HKBA) or outer membrane protein 19 (Omp19), a B. abortus lipoprotein model, induced astrogliosis in mouse striatum. Moreover, infection of astrocytes and microglia with B. abortus induced the secretion of interleukin (IL)-6, IL-1beta, tumor necrosis factor (TNF)-alpha, macrophage chemoattractant protein-1, and KC (CXCL1). HKBA also induced these inflammatory mediators, suggesting the involvement of a structural component of the bacterium. Accordingly, Omp19 induced the same cytokine and chemokine secretion pattern. B. abortus infection induced astrocyte, but not microglia, apoptosis. Indeed, HKBA and Omp19 elicited not only astrocyte apoptosis but also proliferation, two features observed during astrogliosis. Apoptosis induced by HKBA and L-Omp19 was completely suppressed in cells of TNF receptor p55-/- mice or when the general caspase inhibitor Z-VAD-FMK was added to cultures. Hence, TNF-alpha signaling via TNF receptor (TNFR) 1 through the coupling of caspases determines apoptosis. Our results provide proof of the principle that Brucella lipoproteins could be key virulence factors in neurobrucellosis and that astrogliosis might contribute to neurobrucellosis pathogenesis.
Figures
Similar articles
-
Brucella abortus induces TNF-α-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases.J Neuroinflammation. 2013 Apr 12;10:47. doi: 10.1186/1742-2094-10-47. J Neuroinflammation. 2013. PMID: 23587438 Free PMC article.
-
Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus.J Immunol. 2004 Oct 1;173(7):4635-42. doi: 10.4049/jimmunol.173.7.4635. J Immunol. 2004. PMID: 15383598
-
Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases.Infect Immun. 2011 Sep;79(9):3619-32. doi: 10.1128/IAI.05408-11. Epub 2011 Jul 5. Infect Immun. 2011. PMID: 21730088 Free PMC article.
-
Immunopathology of Brucella infection.Recent Pat Antiinfect Drug Discov. 2013 Apr;8(1):18-26. doi: 10.2174/1574891x11308010005. Recent Pat Antiinfect Drug Discov. 2013. PMID: 22812614 Review.
-
The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection.Semin Immunopathol. 2017 Feb;39(2):215-223. doi: 10.1007/s00281-016-0581-1. Epub 2016 Jul 12. Semin Immunopathol. 2017. PMID: 27405866 Free PMC article. Review.
Cited by
-
Brucella abortus Traverses Brain Microvascular Endothelial Cells Using Infected Monocytes as a Trojan Horse.Front Cell Infect Microbiol. 2018 Jun 14;8:200. doi: 10.3389/fcimb.2018.00200. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29963502 Free PMC article.
-
Bystander activation of microglia by Brucella abortus-infected astrocytes induces neuronal death via IL-6 trans-signaling.Front Immunol. 2024 Jan 23;14:1343503. doi: 10.3389/fimmu.2023.1343503. eCollection 2023. Front Immunol. 2024. PMID: 38322014 Free PMC article.
-
Doxycycline Induces Apoptosis of Brucella Suis S2 Strain-Infected HMC3 Microglial Cells by Activating Calreticulin-Dependent JNK/p53 Signaling Pathway.Front Cell Infect Microbiol. 2021 Apr 28;11:640847. doi: 10.3389/fcimb.2021.640847. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33996626 Free PMC article.
-
Cell and Tissue Tropism of Brucella spp.Infect Immun. 2023 May 16;91(5):e0006223. doi: 10.1128/iai.00062-23. Epub 2023 Apr 27. Infect Immun. 2023. PMID: 37129522 Free PMC article. Review.
-
The Role of Astrocytes in CNS Inflammation.Trends Immunol. 2020 Sep;41(9):805-819. doi: 10.1016/j.it.2020.07.007. Epub 2020 Aug 13. Trends Immunol. 2020. PMID: 32800705 Free PMC article. Review.
References
-
- Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995;21:283–289. quiz 290. - PubMed
-
- Goldstein J, Hoffman T, Frasch C, Lizzio EF, Beining PR, Hochstein D, Lee YL, Angus RD, Golding B. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect Immun. 1992;60:1385–1389. - PMC - PubMed
-
- Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol. 2004;173:4635–4642. - PubMed
-
- Zwerdling A, Delpino MV, Barrionuevo P, Cassataro J, Pasquevich KA, Garcia Samartino C, Fossati CA, Giambartolomei GH. Brucella lipoproteins mimic dendritic cell maturation induced by Brucella abortus. Microbes Infect. 2008;10:1346–1354. - PubMed
-
- Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect Immun. 2008;76:250–262. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

