Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells
- PMID: 20093495
- PMCID: PMC2832133
- DOI: 10.2353/ajpath.2010.090956
Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells
Erratum in
- Am J Pathol. 2010 May;176(5):2581
Abstract
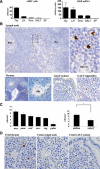
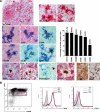
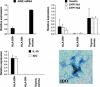
Autoimmune regulator (AIRE) modulates the expression of tissue-restricted antigens (TSAs) and promotes central tolerance in the thymus. However, few autoreactive T cells escape negative selection and reach the periphery, where peripheral tolerance is required to avoid autoimmunity. Murine lymph nodes (LNs) have been shown to contain "stromal" cells expressing AIRE and TSAs. Here we report the occurrence of AIRE-expressing cells in human peripheral lymphoid tissues, including LNs, tonsils, and gut-associated lymphoid tissue, with the exception of the spleen. Notably, AIRE+ cells are absent in fetal LNs and, in postnatal life, they are more numerous in abdominal than in superficial LNs, thus suggesting that their development in periphery may depend on instructive signals from microenvironment and antigen challenge. Extrathymic AIRE+ cells show a dendritic morphology, consistently express human leukocyte antigen-DR (HLADR) and fascin, and are largely positive for CD11c and S100 and for the dendritic cell-activation markers CD40, CD83, DC-LAMP/CD208, and CCR7. Lymphoid, myelomonocytic, mesenchymal, and epithelial cell lineage markers are negative. The HLADRhigh/AIRE+ cell fraction isolated from mesenteric LNs expressed TSAs (insulin, CYP17A1, and CYP21A2), as well as molecules associated with tolerogenic functions, such as interleukin-10 and indoleamine 2,3-dioxygenase. Data indicate that AIRE+ cells in human peripheral lymphoid tissues correspond to a subset of activated interdigitating dendritic cells expressing TSAs and the tolerogenic molecules indoleamine 2,3-dioxygenase and interleukin-10, suggestive of a potential tolerogenic function.
Figures
References
-
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. - PubMed
-
- Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8:304–311. - PubMed
-
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. - PubMed
-
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

