Arhgef1 regulates alpha5beta1 integrin-mediated matrix metalloproteinase expression and is required for homeostatic lung immunity
- PMID: 20093499
- PMCID: PMC2832139
- DOI: 10.2353/ajpath.2010.090200
Arhgef1 regulates alpha5beta1 integrin-mediated matrix metalloproteinase expression and is required for homeostatic lung immunity
Abstract
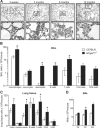
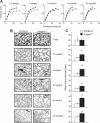
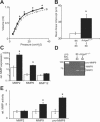
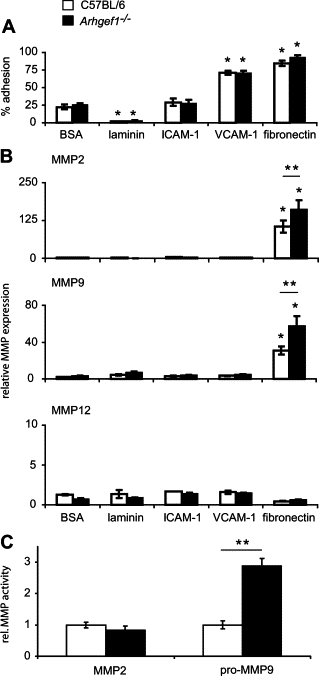
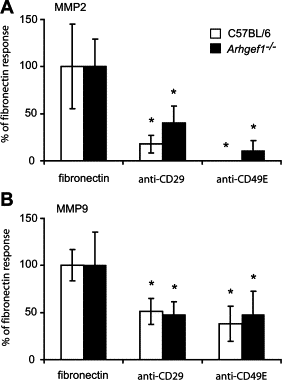
Pulmonary immunity depends on the ability of leukocytes to neutralize potentially harmful and frequent insults to the lung, and appropriate regulation of leukocyte migration and adhesion is integral to this process. Arhgef1 is a hematopoietic-restricted signaling molecule that regulates leukocyte migration and integrin-mediated adhesion. To explore a possible regulatory role for Arhgef1 in pulmonary immunity we examined the lung and its leukocytes in wild-type and Arhgef1-deficient animals. Here we report that the lungs of Arhgef1-/- mice harbored significantly more leukocytes, increased expression and activity of matrix metalloproteinases (MMPs), airspace enlargement, and decreased lung elastance compared with wild-type lungs. Transfer of Arhgef1-/- lung leukocytes to wild-type mice led to airspace enlargement and impaired lung function, indicating that loss of Arhgef1 in leukocytes was sufficient to induce pulmonary pathology. Furthermore, we showed that Arhgef1-deficient peritoneal macrophages when either injected into the lungs of wild-type mice or cultured on fibronectin significantly increased expression and activity of MMPs relative to control macrophages, and the in vitro fibronectin induction was dependent on the alpha5beta1 integrin pair. Together these data demonstrate that Arhgef1 regulates alpha5beta1-mediated MMP expression by macrophages and that loss of Arhgef1 by leukocytes leads to pulmonary pathology.
Figures

Similar articles
-
Thromboxane receptor signaling is required for fibronectin-induced matrix metalloproteinase 9 production by human and murine macrophages and is attenuated by the Arhgef1 molecule.J Biol Chem. 2011 Dec 30;286(52):44521-31. doi: 10.1074/jbc.M111.282772. Epub 2011 Nov 15. J Biol Chem. 2011. PMID: 22086927 Free PMC article.
-
The influence of Arhgef1 on pulmonary leukocyte function.Immunol Res. 2013 Mar;55(1-3):162-6. doi: 10.1007/s12026-012-8360-0. Immunol Res. 2013. PMID: 22941563 Review.
-
Arhgef1 is required by T cells for the development of airway hyperreactivity and inflammation.Am J Respir Crit Care Med. 2007 Jul 1;176(1):10-9. doi: 10.1164/rccm.200702-270OC. Epub 2007 Apr 26. Am J Respir Crit Care Med. 2007. PMID: 17463415 Free PMC article.
-
The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure.Nat Med. 2010 Feb;16(2):183-90. doi: 10.1038/nm.2079. Epub 2010 Jan 24. Nat Med. 2010. PMID: 20098430
-
Modulation of MMPs by cell surface integrin receptor α5β1.Anticancer Agents Med Chem. 2012 Sep;12(7):726-32. doi: 10.2174/187152012802650183. Anticancer Agents Med Chem. 2012. PMID: 22292746 Review.
Cited by
-
Fucoidan Stimulates Monocyte Migration via ERK/p38 Signaling Pathways and MMP9 Secretion.Mar Drugs. 2015 Jun 30;13(7):4156-70. doi: 10.3390/md13074156. Mar Drugs. 2015. PMID: 26133555 Free PMC article.
-
Thromboxane receptor signaling is required for fibronectin-induced matrix metalloproteinase 9 production by human and murine macrophages and is attenuated by the Arhgef1 molecule.J Biol Chem. 2011 Dec 30;286(52):44521-31. doi: 10.1074/jbc.M111.282772. Epub 2011 Nov 15. J Biol Chem. 2011. PMID: 22086927 Free PMC article.
-
Cigarette smoke inhibits ROCK2 activation in T cells and modulates IL-22 production.Mol Immunol. 2016 Mar;71:115-122. doi: 10.1016/j.molimm.2016.01.013. Epub 2016 Feb 13. Mol Immunol. 2016. PMID: 26882474 Free PMC article.
-
Integrative multiomics and in silico analysis revealed the role of ARHGEF1 and its screened antagonist in mild and severe COVID-19 patients.J Cell Biochem. 2022 Mar;123(3):673-690. doi: 10.1002/jcb.30213. Epub 2022 Jan 17. J Cell Biochem. 2022. PMID: 35037717 Free PMC article.
-
The influence of Arhgef1 on pulmonary leukocyte function.Immunol Res. 2013 Mar;55(1-3):162-6. doi: 10.1007/s12026-012-8360-0. Immunol Res. 2013. PMID: 22941563 Review.
References
-
- Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. - PubMed
-
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. - PubMed
-
- Henderson WR, Jr, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. - PMC - PubMed
-
- Paine R, 3rd, Morris SB, Jin H, Baleeiro CE, Wilcoxen SE. ICAM-1 facilitates alveolar macrophage phagocytic activity through effects on migration over the AEC surface. Am J Physiol. 2002;283:L180–L187. - PubMed
-
- Kranenburg AR, Willems-Widyastuti A, Moori WJ, Sterk PJ, Alagappan VK, de Boer WI, Sharma HS. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;126:725–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

