MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot-Marie-tooth 1A neuropathy
- PMID: 20093502
- PMCID: PMC2832158
- DOI: 10.2353/ajpath.2010.090694
MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot-Marie-tooth 1A neuropathy
Abstract
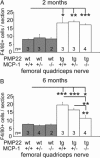
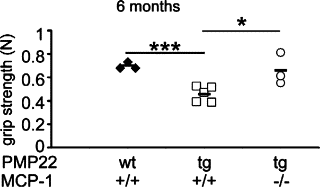
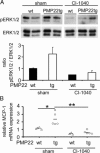
Charcot-Marie-Tooth 1A (CMT1A) neuropathy, the most common inherited peripheral neuropathy, is primarily caused by a gene duplication for the peripheral myelin protein-22 (PMP22). In an accordant mouse model, we investigated the role of monocyte chemoattractant protein-1 (MCP-1/CCL2) as a regulator of nerve macrophages and neural damage including axonopathy and demyelination. By generating PMP22tg mice with reduced levels or lack of MCP-1/CCL2, we found that MCP-1/CCL2 is involved in the increase of macrophages in mutant nerves. PMP22tg mice with wild-type levels of MCP-1/CCL2 showed strong macrophage increase in the diseased nerves, whereas either 50% reduction or total absence of MCP-1/CCL2 led to a moderate or a strong reduction of nerve macrophages, respectively. Interestingly, MCP-1/CCL2 expression level and macrophage numbers were correlated with features indicative of axon damage, such as maldistribution of K+ channels, reduced compound muscle action potentials, and muscle weakness. Demyelinating features, however, were most highly reduced when MCP-1/CCL2 was diminished by 50%, whereas complete lack of MCP-1/CCL2 showed an intermediate demyelinating phenotype. We also identified the MEK1/2-ERK1/2-pathway as being involved in MCP-1/CCL2 expression in the Schwann cells of the CMT1A model. Our data show that, in a CMT1A model, MCP-1/CCL2 activates nerve macrophages, mediates both axon damage and demyelination, and may thus be a promising target for therapeutic approaches.
Figures



Similar articles
-
Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X.Hum Mol Genet. 2010 Sep 15;19(18):3530-43. doi: 10.1093/hmg/ddq269. Epub 2010 Jun 30. Hum Mol Genet. 2010. PMID: 20591826
-
An essential role of MAG in mediating axon-myelin attachment in Charcot-Marie-Tooth 1A disease.Neurobiol Dis. 2013 Jan;49:221-31. doi: 10.1016/j.nbd.2012.08.009. Epub 2012 Aug 25. Neurobiol Dis. 2013. PMID: 22940629 Free PMC article.
-
Evidence for macrophage-mediated myelin disruption in an animal model for Charcot-Marie-Tooth neuropathy type 1A.J Neurosci Res. 2005 Sep 15;81(6):857-64. doi: 10.1002/jnr.20601. J Neurosci Res. 2005. PMID: 16041800
-
Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients.Brain. 2003 Jan;126(Pt 1):134-51. doi: 10.1093/brain/awg012. Brain. 2003. PMID: 12477701 Review.
-
Comparison of a new pmp22 transgenic mouse line with other mouse models and human patients with CMT1A.J Anat. 2002 Apr;200(4):377-90. doi: 10.1046/j.1469-7580.2002.00039.x. J Anat. 2002. PMID: 12090404 Free PMC article. Review.
Cited by
-
After Nf1 loss in Schwann cells, inflammation drives neurofibroma formation.Neurooncol Adv. 2019 Nov 22;2(Suppl 1):i23-i32. doi: 10.1093/noajnl/vdz045. eCollection 2020 Jul. Neurooncol Adv. 2019. PMID: 32642730 Free PMC article. Review.
-
Sustained MAPK/ERK Activation in Adult Schwann Cells Impairs Nerve Repair.J Neurosci. 2018 Jan 17;38(3):679-690. doi: 10.1523/JNEUROSCI.2255-17.2017. Epub 2017 Dec 7. J Neurosci. 2018. PMID: 29217688 Free PMC article.
-
Pharmacologic Targeting of the C-Terminus of Heat Shock Protein 90 Improves Neuromuscular Function in Animal Models of Charcot Marie Tooth X1 Disease.ACS Pharmacol Transl Sci. 2023 Jan 20;6(2):306-319. doi: 10.1021/acsptsci.2c00223. eCollection 2023 Feb 10. ACS Pharmacol Transl Sci. 2023. PMID: 36798471 Free PMC article.
-
Biochemical characterization of protein quality control mechanisms during disease progression in the C22 mouse model of CMT1A.ASN Neuro. 2013 Dec 3;5(5):e00128. doi: 10.1042/AN20130024. ASN Neuro. 2013. PMID: 24175617 Free PMC article.
-
Dysregulation of ErbB Receptor Trafficking and Signaling in Demyelinating Charcot-Marie-Tooth Disease.Mol Neurobiol. 2017 Jan;54(1):87-100. doi: 10.1007/s12035-015-9668-2. Epub 2016 Jan 5. Mol Neurobiol. 2017. PMID: 26732592 Free PMC article. Review.
References
-
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. - PubMed
-
- Nave KA, Sereda MW, Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies–from basic to clinical research. Nat Clin Pract Neurol. 2007;3:453–464. - PubMed
-
- Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. - PubMed
-
- Sereda MW, Meyer zu Horste G, Suter U, Uzma N, Nave KA. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat Med. 2003;9:1533–1537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

