Transmural dispersion of repolarization in failing and nonfailing human ventricle
- PMID: 20093630
- PMCID: PMC2842469
- DOI: 10.1161/CIRCRESAHA.109.204891
Transmural dispersion of repolarization in failing and nonfailing human ventricle
Abstract
Rationale: Transmural dispersion of repolarization has been shown to play a role in the genesis of ventricular tachycardia and fibrillation in different animal models of heart failure (HF). Heterogeneous changes of repolarization within the midmyocardial population of ventricular cells have been considered an important contributor to the HF phenotype. However, there is limited electrophysiological data from the human heart.
Objective: To study electrophysiological remodeling of transmural repolarization in the failing and nonfailing human hearts.
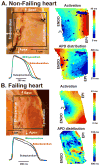
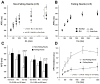
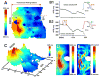
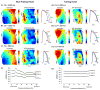
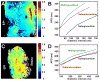
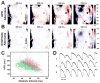
Methods and results: We optically mapped the action potential duration (APD) in the coronary-perfused scar-free posterior-lateral left ventricular free wall wedge preparations from failing (n=5) and nonfailing (n=5) human hearts. During slow pacing (S1S1=2000 ms), in the nonfailing hearts we observed significant transmural APD gradient: subepicardial, midmyocardial, and subendocardial APD80 were 383+/-21, 455+/-20, and 494+/-22 ms, respectively. In 60% of nonfailing hearts (3 of 5), we found midmyocardial islands of cells that presented a distinctly long APD (537+/-40 ms) and a steep local APD gradient (27+/-7 ms/mm) compared with the neighboring myocardium. HF resulted in prolongation of APD80: 477+/-22 ms, 495+/-29 ms, and 506+/-35 ms for the subepi-, mid-, and subendocardium, respectively, while reducing transmural APD80 difference from 111+/-13 to 29+/-6 ms (P<0.005) and presence of any prominent local APD gradient. In HF, immunostaining revealed a significant reduction of connexin43 expression on the subepicardium.
Conclusions: We present for the first time direct experimental evidence of a transmural APD gradient in the human heart. HF results in the heterogeneous prolongation of APD, which significantly reduces the transmural and local APD gradients.
Figures


Comment in
-
M cells in the human heart.Circ Res. 2010 Mar 19;106(5):815-7. doi: 10.1161/CIRCRESAHA.109.216226. Circ Res. 2010. PMID: 20299671 Free PMC article. No abstract available.
-
Does APD gradient indicate dispersion of repolarization ?Circ Res. 2010 Aug 6;107(3):e9; author reply e10. doi: 10.1161/CIRCRESAHA.110.224048. Circ Res. 2010. PMID: 20689067 No abstract available.
References
-
- Jin H, Lyon AR, Akar FG. Arrhythmia mechanisms in the failing heart. Pacing Clin Electrophysiol. 2008;31:1048–1056. - PubMed
-
- Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. - PubMed
-
- Nass RD, Aiba T, Tomaselli GF, Akar FG. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med. 2008;5:196–207. - PubMed
-
- Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–645. - PubMed
-
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

