Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis
- PMID: 20093631
- PMCID: PMC2847792
- DOI: 10.1161/CIRCRESAHA.109.198069
Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis
Abstract
Rationale: Multiple protein kinases have been implicated in cardiovascular disease; however, little is known about the role of their counterparts: the protein phosphatases.
Objective: To test the hypothesis that mitogen-activated protein kinase phosphatase (MKP)-1 is actively involved in atherogenesis.
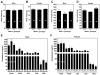
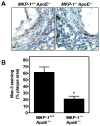
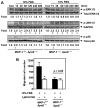
Methods and results: Mice with homozygous deficiency in MKP-1 (MKP-1(-/-)) were bred with apolipoprotein (Apo)E-deficient mice (ApoE(-/-)) and the 3 MKP-1 genotypes (MKP-1(+/+)/ApoE(-/-) ; MKP-1(+/-)/ApoE(-/-) and MKP-1(-/-)/ApoE(-/-)) were maintained on a normal chow diet for 16 weeks. The 3 groups of mice exhibited similar body weight and serum lipid profiles; however, both MKP-1(+/-) and MKP-1(-/-) mice had significantly less aortic root atherosclerotic lesion formation than MKP-1(+/+) mice. Less en face lesion was observed in 8-month-old MKP-1(-/-) mice. The reduction in atherosclerosis was accompanied by decreased plasma levels of interleukin-1alpha and tumor necrosis factor alpha, and preceded by increased antiinflammatory cytokine interleukin-10. In addition, MKP-1-null mice had higher levels of plasma stromal cell-derived factor-1a, which negatively correlated with atherosclerotic lesion size. Immunohistochemical analysis revealed that MKP-1 expression was enriched in macrophage-rich areas versus smooth muscle cell regions of the atheroma. Furthermore, macrophages isolated from MKP-1-null mice showed dramatic defects in their spreading/migration and impairment in extracellular signal-regulated kinase, but not c-Jun N-terminal kinase and p38, pathway activation. In line with this, MKP-1-null atheroma exhibited less macrophage content. Finally, transplantation of MKP-1-intact bone marrow into MKP-1-null mice fully rescued the wild-type atherosclerotic phenotype.
Conclusion: These findings demonstrate that chronic deficiency of MKP-1 leads to decreased atherosclerosis via mechanisms involving impaired macrophage migration and defective extracellular signal-regulated kinase signaling.
Figures






Similar articles
-
MicroRNA-21 deficiency attenuated atherogenesis and decreased macrophage infiltration by targeting Dusp-8.Atherosclerosis. 2019 Dec;291:78-86. doi: 10.1016/j.atherosclerosis.2019.10.003. Epub 2019 Oct 11. Atherosclerosis. 2019. PMID: 31704554
-
Mitogen-activated protein kinase phosphatase-1 deficiency decreases atherosclerosis in apolipoprotein E null mice by reducing monocyte chemoattractant protein-1 levels.Mol Genet Metab. 2010 Sep;101(1):66-75. doi: 10.1016/j.ymgme.2010.05.009. Epub 2010 Jun 9. Mol Genet Metab. 2010. PMID: 20619710 Free PMC article.
-
Potential role for mitogen-activated protein kinase phosphatase-1 in the development of atherosclerotic lesions in mouse models.Arterioscler Thromb Vasc Biol. 2004 Sep;24(9):1676-81. doi: 10.1161/01.ATV.0000138342.94314.64. Epub 2004 Jul 8. Arterioscler Thromb Vasc Biol. 2004. PMID: 15242861
-
Vav Guanine nucleotide exchange factors regulate atherosclerotic lesion development in mice.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2053-7. doi: 10.1161/ATVBAHA.113.301414. Epub 2013 Jul 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23825362 Free PMC article.
-
Acetylation of MKP-1 and the control of inflammation.Sci Signal. 2008 Oct 14;1(41):pe44. doi: 10.1126/scisignal.141pe44. Sci Signal. 2008. PMID: 18922786 Free PMC article. Review.
Cited by
-
Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment.Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2803-12. doi: 10.1073/pnas.1212596109. Epub 2012 Sep 18. Proc Natl Acad Sci U S A. 2012. PMID: 22991462 Free PMC article.
-
Modified LDL Particles Activate Inflammatory Pathways in Monocyte-derived Macrophages: Transcriptome Analysis.Curr Pharm Des. 2018;24(26):3143-3151. doi: 10.2174/1381612824666180911120039. Curr Pharm Des. 2018. PMID: 30205792 Free PMC article.
-
JNK and cardiometabolic dysfunction.Biosci Rep. 2019 Jul 18;39(7):BSR20190267. doi: 10.1042/BSR20190267. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31270248 Free PMC article. Review.
-
Mitogen-activated protein kinase phosphatase 1 (MKP-1) in macrophage biology and cardiovascular disease. A redox-regulated master controller of monocyte function and macrophage phenotype.Free Radic Biol Med. 2017 Aug;109:75-83. doi: 10.1016/j.freeradbiomed.2017.03.020. Epub 2017 Mar 19. Free Radic Biol Med. 2017. PMID: 28330703 Free PMC article. Review.
-
The P2Y2 receptor mediates terminal adipocyte differentiation and insulin resistance: Evidence for a dual G-protein coupling mode.J Biol Chem. 2024 Feb;300(2):105589. doi: 10.1016/j.jbc.2023.105589. Epub 2023 Dec 21. J Biol Chem. 2024. PMID: 38141758 Free PMC article.
References
-
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
-
- Senokuchi T, Matsumura T, Sakai M, Matsuo T, Yano M, Kiritoshi S, Sonoda K, Kukidome D, Nishikawa T, Araki E. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase mediate macrophage proliferation induced by oxidized low-density lipoprotein. Atherosclerosis. 2004;176:233–245. - PubMed
-
- DiCorleto PE. Protein tyrosine phosphatases in the vessel wall: counterpoint to the tyrosine kinases. Arterioscler Thromb Vasc Biol. 2000;20:1179–1181. - PubMed
-
- Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemée A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol. 2007;8:234–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

