Neutrophil alpha-defensins cause lung injury by disrupting the capillary-epithelial barrier
- PMID: 20093642
- PMCID: PMC2862305
- DOI: 10.1164/rccm.200907-1128OC
Neutrophil alpha-defensins cause lung injury by disrupting the capillary-epithelial barrier
Abstract
Rationale: The involvement of neutrophil activation in the sentinel, potentially reversible, events in the pathogenesis of acute lung injury (ALI) is only partially understood. alpha-Defensins are the most abundant proteins secreted by activated human neutrophils, but their contribution to ALI in mouse models is hindered by their absence from murine neutrophils and the inability to study their effects in isolation in other species.
Objectives: To study the role of alpha-defensins in the pathogenesis of ALI in a clinically relevant setting using mice transgenic for polymorphonuclear leukocyte expression of alpha-defensins.
Methods: Transgenic mice expressing polymorphonuclear leukocyte alpha-defensins were generated. ALI was induced by acid aspiration. Pulmonary vascular permeability was studied in vivo using labeled dextran and fibrin deposition. The role of the low-density lipoprotein-related receptor (LRP) in permeability was examined.
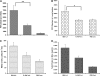
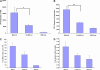
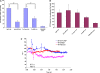
Measurements and main results: Acid aspiration induced neutrophil migration and release of alpha-defensins into lung parenchyma and airways. ALI was more severe in alpha-defensin-expressing mice than in wild-type mice, as determined by inspection, influx of neutrophils into the interstitial space and airways, histological evidence of epithelial injury, interstitial edema, extravascular fibrin deposition, impaired oxygenation, and reduced survival. Within 4 hours of insult, alpha-defensin-expressing mice showed greater disruption of capillary-epithelial barrier function and ALI that was attenuated by systemic or intratracheal administration of specific inhibitors of the LRP.
Conclusions: alpha-Defensins mediate ALI through LRP-mediated loss of capillary-epithelial barrier function, suggesting a potential new approach to intervention.
Figures

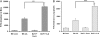


Similar articles
-
Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury.Am J Respir Crit Care Med. 2012 Mar 15;185(6):628-36. doi: 10.1164/rccm.201108-1533OC. Epub 2012 Jan 12. Am J Respir Crit Care Med. 2012. PMID: 22246174 Free PMC article.
-
Rhesus θ-Defensin-1 Attenuates Endotoxin-induced Acute Lung Injury by Inhibiting Proinflammatory Cytokines and Neutrophil Recruitment.Am J Respir Cell Mol Biol. 2018 Mar;58(3):310-319. doi: 10.1165/rcmb.2016-0428OC. Am J Respir Cell Mol Biol. 2018. PMID: 28954201 Free PMC article.
-
Platelet-Specific Chemokines Contribute to the Pathogenesis of Acute Lung Injury.Am J Respir Cell Mol Biol. 2017 Feb;56(2):261-270. doi: 10.1165/rcmb.2015-0245OC. Am J Respir Cell Mol Biol. 2017. PMID: 27755915 Free PMC article.
-
Neutrophils in acute lung injury.Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2278-83. doi: 10.2741/4051. Front Biosci (Landmark Ed). 2012. PMID: 22652778 Review.
-
Regulation and repair of the alveolar-capillary barrier in acute lung injury.Annu Rev Physiol. 2013;75:593-615. doi: 10.1146/annurev-physiol-030212-183756. Annu Rev Physiol. 2013. PMID: 23398155 Review.
Cited by
-
Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury.Am J Respir Crit Care Med. 2012 Mar 15;185(6):628-36. doi: 10.1164/rccm.201108-1533OC. Epub 2012 Jan 12. Am J Respir Crit Care Med. 2012. PMID: 22246174 Free PMC article.
-
Establishment of a Predictive Model for Acute Respiratory Distress Syndrome in Patients with Bacterial Pneumonia.J Inflamm Res. 2024 May 8;17:2825-2834. doi: 10.2147/JIR.S458690. eCollection 2024. J Inflamm Res. 2024. PMID: 38737109 Free PMC article.
-
α-Defensins Induce a Post-translational Modification of Low Density Lipoprotein (LDL) That Promotes Atherosclerosis at Normal Levels of Plasma Cholesterol.J Biol Chem. 2016 Feb 5;291(6):2777-86. doi: 10.1074/jbc.M115.669812. Epub 2015 Oct 30. J Biol Chem. 2016. PMID: 26518877 Free PMC article.
-
TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury.J Immunol. 2013 Apr 15;190(8):4274-82. doi: 10.4049/jimmunol.1202437. Epub 2013 Mar 13. J Immunol. 2013. PMID: 23487422 Free PMC article.
-
Pulmonary permeability assessed by fluorescent-labeled dextran instilled intranasally into mice with LPS-induced acute lung injury.PLoS One. 2014 Jul 9;9(7):e101925. doi: 10.1371/journal.pone.0101925. eCollection 2014. PLoS One. 2014. PMID: 25007191 Free PMC article.
References
-
- Matthay MAG, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Hoffman E, Hubmayr M, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. - PubMed
-
- Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003;124:328–336. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. - PubMed
-
- Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 1999;281:354–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

