Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus
- PMID: 20093660
- PMCID: PMC3774512
- DOI: 10.1152/japplphysiol.01240.2009
Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus
Abstract
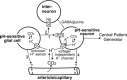
Central chemoreception is the mechanism by which CO2/pH-sensitive neurons (i.e., chemoreceptors) regulate breathing in response to changes in tissue pH. A region of the brain stem called the retrotrapezoid nucleus (RTN) is thought to be an important site of chemoreception (23), and recent evidence suggests that RTN chemoreception involves two interrelated mechanisms: H+-mediated activation of pH-sensitive neurons (38) and purinergic signaling (19), possibly from pH-sensitive glial cells. A third, potentially important, aspect of RTN chemoreception is the regulation of blood flow, which is an important determinate of tissue pH and consequently chemoreceptor activity. It is well established in vivo that changes in cerebral blood flow can profoundly affect the chemoreflex (2); e.g., limiting blood flow by vasoconstriction acidifies tissue pH and increases the ventilatory response to CO2, whereas vasodilation can wash out metabolically produced CO2 from tissue to increase tissue pH and decrease the stimulus at chemoreceptors. In this review, we will summarize the defining characteristics of pH-sensitive neurons and discuss potential contributions of pH-sensitive glial cells as both a source of purinergic drive to pH-sensitive neurons and a modulator of vasculature tone.
Figures

Similar articles
-
Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling.J Physiol. 2012 May 1;590(9):2137-50. doi: 10.1113/jphysiol.2012.229666. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411009 Free PMC article.
-
Bicarbonate directly modulates activity of chemosensitive neurons in the retrotrapezoid nucleus.J Physiol. 2018 Sep;596(17):4033-4042. doi: 10.1113/JP276104. Epub 2018 Jul 5. J Physiol. 2018. PMID: 29873079 Free PMC article.
-
Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism.J Neurophysiol. 2010 Dec;104(6):3042-52. doi: 10.1152/jn.00544.2010. Epub 2010 Oct 6. J Neurophysiol. 2010. PMID: 20926613 Free PMC article.
-
Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive.Exp Physiol. 2011 Apr;96(4):400-6. doi: 10.1113/expphysiol.2010.053140. Epub 2010 Dec 17. Exp Physiol. 2011. PMID: 21169332 Free PMC article. Review.
-
Update on vascular control of central chemoreceptors.Exp Physiol. 2024 Nov;109(11):1837-1843. doi: 10.1113/EP091329. Epub 2023 Dec 28. Exp Physiol. 2024. PMID: 38153366 Free PMC article. Review.
Cited by
-
The psychophysiology of the sigh: I: The sigh from the physiological perspective.Biol Psychol. 2022 Apr;170:108313. doi: 10.1016/j.biopsycho.2022.108313. Epub 2022 Mar 11. Biol Psychol. 2022. PMID: 35288214 Free PMC article. Review.
-
Central chemoreceptors: locations and functions.Compr Physiol. 2012 Jan;2(1):221-54. doi: 10.1002/cphy.c100083. Compr Physiol. 2012. PMID: 23728974 Free PMC article. Review.
-
Regulation of breathing and autonomic outflows by chemoreceptors.Compr Physiol. 2014 Oct;4(4):1511-62. doi: 10.1002/cphy.c140004. Compr Physiol. 2014. PMID: 25428853 Free PMC article. Review.
-
Central CO2 chemoreception in cardiorespiratory control.J Appl Physiol (1985). 2010 Apr;108(4):976-8. doi: 10.1152/japplphysiol.00133.2010. Epub 2010 Feb 11. J Appl Physiol (1985). 2010. PMID: 20150571 Free PMC article. No abstract available.
-
Proton pump inhibition increases rapid eye movement sleep in the rat.Biomed Res Int. 2014;2014:162314. doi: 10.1155/2014/162314. Epub 2014 Feb 19. Biomed Res Int. 2014. PMID: 24701564 Free PMC article.
References
-
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 - PubMed
-
- Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci 12: 2049–2058, 2000 - PubMed
-
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008 - PMC - PubMed
-
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

