Antibody fragments: hope and hype
- PMID: 20093855
- PMCID: PMC2828581
- DOI: 10.4161/mabs.2.1.10786
Antibody fragments: hope and hype
Abstract
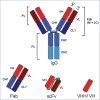
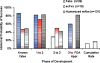
The antibody molecule is modular and separate domains can be extracted through biochemical or genetic means. It is clear from review of the literature that a wave of novel, antigen-specific molecular forms may soon enter clinical evaluation. This report examines the developmental histories of therapeutics derived from antigen-specific fragments of antibodies produced by recombinant processes. Three general types of fragments were observed, antigen-binding fragments (Fab), single chain variable fragments (scFv) and "third generation" (3G), each representing a successive wave of antibody fragment technology. In parallel, drug developers have explored multi-specificity and conjugation with exogenous functional moieties in all three fragment types. Despite high hopes and an active pipeline, enthusiasm for differentiating performance of fragments should, perhaps, be tempered as there are yet few data that suggest these molecules have distinct clinical properties due only to their size.
Figures
Similar articles
-
Recombinant antibody fragments and immunotoxin fusions for cancer therapy.In Vivo. 2000 Jan-Feb;14(1):21-7. In Vivo. 2000. PMID: 10757057 Review.
-
A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells.J Immunother. 2008 Nov-Dec;31(9):871-84. doi: 10.1097/CJI.0b013e318186c8b4. J Immunother. 2008. PMID: 18833000
-
Pharmacokinetics and biodistribution of genetically-engineered antibodies.Q J Nucl Med. 1998 Dec;42(4):225-41. Q J Nucl Med. 1998. PMID: 9973838 Review.
-
A novel bispecific tetravalent antibody fusion protein to target costimulatory activity for T-cell activation to tumor cells overexpressing ErbB2/HER2.J Mol Biol. 2005 Mar 11;346(5):1299-311. doi: 10.1016/j.jmb.2004.12.052. Epub 2005 Jan 22. J Mol Biol. 2005. PMID: 15713482
-
Fab-scFv fusion protein: an efficient approach to production of bispecific antibody fragments.J Immunol Methods. 2002 Sep 15;267(2):213-26. doi: 10.1016/s0022-1759(02)00148-5. J Immunol Methods. 2002. PMID: 12165442
Cited by
-
Development and in vitro characterization of a humanized scFv against fungal infections.PLoS One. 2022 Oct 31;17(10):e0276786. doi: 10.1371/journal.pone.0276786. eCollection 2022. PLoS One. 2022. PMID: 36315567 Free PMC article.
-
Separation of closely related monoclonal antibody charge variant impurities using poly(ethylenimine)-grafted cation-exchange chromatography resin.3 Biotech. 2022 Nov;12(11):293. doi: 10.1007/s13205-022-03350-9. Epub 2022 Sep 27. 3 Biotech. 2022. PMID: 36276450 Free PMC article.
-
Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition.Front Immunol. 2018 Oct 16;9:2278. doi: 10.3389/fimmu.2018.02278. eCollection 2018. Front Immunol. 2018. PMID: 30386328 Free PMC article. Review.
-
The mosaic puzzle of the therapeutic monoclonal antibodies and antibody fragments - A modular transition from full-length immunoglobulins to antibody mimetics.Leuk Res Rep. 2022 Jun 27;18:100335. doi: 10.1016/j.lrr.2022.100335. eCollection 2022. Leuk Res Rep. 2022. PMID: 35832747 Free PMC article.
-
HTRF: a technology tailored for biomarker determination-novel analytical detection system suitable for detection of specific autoimmune antibodies as biomarkers in nanogram level in different body fluids.EPMA J. 2015 Nov 27;6:23. doi: 10.1186/s13167-015-0046-y. eCollection 2015. EPMA J. 2015. PMID: 26617680 Free PMC article.
References
-
- Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat Biotechnol. 2009;27:331–337. - PubMed
-
- Pappas DA, Bathon JM, Hanicq D, Yasothan U, Kirkpatrick P. Golimumab. Nat Rev Drug Discov. 2009;8:695–696. - PubMed
-
- Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–3408. - PubMed
-
- Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:814–819. - PubMed
-
- Cumber AJ, Ward ES, Winter G, Parnell GD, Wawrzynczak EJ. Comparative stabilities in vitro and in vivo of a recombinant mouse antibody FvCys fragment and a bisFvCys conjugate. J Immunol. 1992;149:120–126. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
