Omega-3 fatty acid supplementation to prevent recurrent preterm birth: a randomized controlled trial
- PMID: 20093894
- PMCID: PMC4022705
- DOI: 10.1097/AOG.0b013e3181cbd60e
Omega-3 fatty acid supplementation to prevent recurrent preterm birth: a randomized controlled trial
Abstract
Objective: To assess whether the addition of an omega-3 long-chain polyunsaturated fatty acid supplement would reduce preterm birth in women with at least one prior spontaneous preterm birth receiving 17alpha-hydroxyprogesterone caproate.
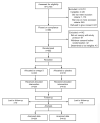
Methods: We conducted a randomized, double-masked, placebo-controlled trial in 13 centers. Women with a history of prior spontaneous singleton preterm birth and a current singleton gestation were assigned to either a daily omega-3 supplement (1,200 mg eicosapentaenoic acid and 800 mg docosahexaenoic acid) or matching placebo from 16-22 through 36 weeks of gestation. All participants received weekly intramuscular 17alpha-hydroxyprogesterone caproate (250 mg). The primary study outcome was delivery before 37 weeks of gestation. A sample size of 800 was necessary to have 80% power to detect a 30% reduction in the primary outcome from 30%, assuming a type I error two-sided of 5%.
Results: A total of 852 women were included, and none was lost to follow up. Delivery before 37 weeks of gestation occurred in 37.8% (164/434) of women in the omega-3 group and 41.6% (174/418) in the placebo group (relative risk 0.91, 95% confidence interval 0.77-1.07).
Conclusion: Omega-3 long-chain polyunsaturated fatty acid supplementation offered no benefit in reducing preterm birth among women receiving 17alpha-hydroxyprogesterone caproate who have a history of preterm delivery.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00135902.
Level of evidence: I.
Figures

References
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. - PubMed
-
- Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep. 2003;52:1–85. - PubMed
-
- Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):7–16. - PubMed
-
- Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178:1035–40. - PubMed
-
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD019897/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- MO1-RR-000080/RR/NCRR NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

