POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation
- PMID: 20094033
- PMCID: PMC2837173
- DOI: 10.1038/emboj.2009.409
POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation
Abstract
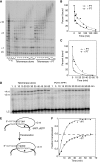
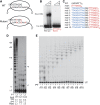
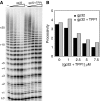
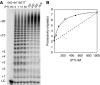
Telomerase contributes to chromosome end replication by synthesizing repeats of telomeric DNA, and the telomeric DNA-binding proteins protection of telomeres (POT1) and TPP1 synergistically increase its repeat addition processivity. To understand the mechanism of increased processivity, we measured the effect of POT1-TPP1 on individual steps in the telomerase reaction cycle. Under conditions where telomerase was actively synthesizing DNA, POT1-TPP1 bound to the primer decreased primer dissociation rate. In addition, POT1-TPP1 increased the translocation efficiency. A template-mutant telomerase that synthesizes DNA that cannot be bound by POT1-TPP1 exhibited increased processivity only when the primer contained at least one POT1-TPP1-binding site, so a single POT1-TPP1-DNA interaction is necessary and sufficient for stimulating processivity. The POT1-TPP1 effect is specific, as another single-stranded DNA-binding protein, gp32, cannot substitute. POT1-TPP1 increased processivity even when substoichiometric relative to the DNA, providing evidence for a recruitment function. These results support a model in which POT1-TPP1 enhances telomerase processivity in a manner markedly different from the sliding clamps used by DNA polymerases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Functional interaction between telomere protein TPP1 and telomerase.Genes Dev. 2010 Mar 15;24(6):613-22. doi: 10.1101/gad.1881810. Genes Dev. 2010. PMID: 20231318 Free PMC article.
-
TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase.Nature. 2007 Feb 1;445(7127):559-62. doi: 10.1038/nature05469. Epub 2007 Jan 21. Nature. 2007. PMID: 17237767
-
The POT1-TPP1 telomere complex is a telomerase processivity factor.Nature. 2007 Feb 1;445(7127):506-10. doi: 10.1038/nature05454. Epub 2007 Jan 21. Nature. 2007. PMID: 17237768
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
Cited by
-
Telomere maintenance in African trypanosomes.Front Mol Biosci. 2023 Nov 24;10:1302557. doi: 10.3389/fmolb.2023.1302557. eCollection 2023. Front Mol Biosci. 2023. PMID: 38074093 Free PMC article. Review.
-
The Telomere-Telomerase System Is Detrimental to Health at High-Altitude.Int J Environ Res Public Health. 2023 Jan 20;20(3):1935. doi: 10.3390/ijerph20031935. Int J Environ Res Public Health. 2023. PMID: 36767300 Free PMC article.
-
Structure of active human telomerase with telomere shelterin protein TPP1.Nature. 2022 Apr;604(7906):578-583. doi: 10.1038/s41586-022-04582-8. Epub 2022 Apr 13. Nature. 2022. PMID: 35418675 Free PMC article.
-
Interaction hub critical for telomerase recruitment and primer-template handling for catalysis.Life Sci Alliance. 2023 Mar 24;6(6):e202201727. doi: 10.26508/lsa.202201727. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36963832 Free PMC article.
-
Telomeres in Plants and Humans: Not So Different, Not So Similar.Cells. 2019 Jan 16;8(1):58. doi: 10.3390/cells8010058. Cells. 2019. PMID: 30654521 Free PMC article. Review.
References
-
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 - PubMed
-
- Bryan TM, Goodrich KJ, Cech TR (2000) A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J Biol Chem 275: 24199–24207 - PubMed
-
- Cristofari G, Adolf E, Reichenbach P, Sikora K, Terns RM, Terns MP, Lingner J (2007a) Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol Cell 27: 882–889 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

