POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation
- PMID: 20094033
- PMCID: PMC2837173
- DOI: 10.1038/emboj.2009.409
POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation
Abstract
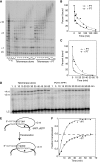
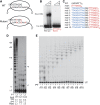
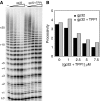
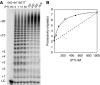
Telomerase contributes to chromosome end replication by synthesizing repeats of telomeric DNA, and the telomeric DNA-binding proteins protection of telomeres (POT1) and TPP1 synergistically increase its repeat addition processivity. To understand the mechanism of increased processivity, we measured the effect of POT1-TPP1 on individual steps in the telomerase reaction cycle. Under conditions where telomerase was actively synthesizing DNA, POT1-TPP1 bound to the primer decreased primer dissociation rate. In addition, POT1-TPP1 increased the translocation efficiency. A template-mutant telomerase that synthesizes DNA that cannot be bound by POT1-TPP1 exhibited increased processivity only when the primer contained at least one POT1-TPP1-binding site, so a single POT1-TPP1-DNA interaction is necessary and sufficient for stimulating processivity. The POT1-TPP1 effect is specific, as another single-stranded DNA-binding protein, gp32, cannot substitute. POT1-TPP1 increased processivity even when substoichiometric relative to the DNA, providing evidence for a recruitment function. These results support a model in which POT1-TPP1 enhances telomerase processivity in a manner markedly different from the sliding clamps used by DNA polymerases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 - PubMed
-
- Bryan TM, Goodrich KJ, Cech TR (2000) A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J Biol Chem 275: 24199–24207 - PubMed
-
- Cristofari G, Adolf E, Reichenbach P, Sikora K, Terns RM, Terns MP, Lingner J (2007a) Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol Cell 27: 882–889 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

