Functional characterization of tlmH in Streptoalloteichus hindustanus E465-94 ATCC 31158 unveiling new insight into tallysomycin biosynthesis and affording a novel bleomycin analog
- PMID: 20094654
- PMCID: PMC3082865
- DOI: 10.1039/b918106g
Functional characterization of tlmH in Streptoalloteichus hindustanus E465-94 ATCC 31158 unveiling new insight into tallysomycin biosynthesis and affording a novel bleomycin analog
Abstract
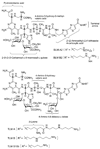
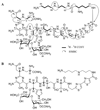
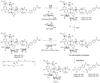
Tallysomycins (TLMs) belong to the bleomycin (BLM) family of anticancer antibiotics and differ from the BLMs principally by the presence of a 4-amino-4,6-dideoxy-L-talose attached to C-41 of the TLM backbone as part of a glycosylcarbinolamide. To facilitate an understanding of the differences in anticancer activities observed between TLMs and BLMs, we thought to generate des-talose TLM analogs by engineering TLM biosynthesis in Streptoalloteichus hindustanus E465-94 ATCC 31158. Here we report (i) the engineering of the DeltatlmH mutant SB8005 strain that produces the two TLM analogs, TLM H-1 and TLM H-2, (ii) production, isolation, and structural elucidation of TLM H-1 and TLM H-2 by NMR and mass spectroscopic analyses as the desired des-talose TLM analogs, and (iii) comparison of the DNA cleavage activities of TLM H-1 with selected TLMs and BLMs. These findings support the previous functional assignment of tlmH to encode an alpha-ketoglutarate-dependent hydroxylase and unveil the TlmH-catalyzed hydroxylation at both C-41 and C-42 and the TlmK-catalyzed glycosylation of a labile carbinolamide intermediate as the final two steps for TLM biosynthesis. TlmH is apparently distinct from other enzymes known to catalyze carbinolamide formation. The availability of TLM H-1 now sets the stage to study the TlmH enzymology in vitro and to elucidate the exact contribution of the l-talose to the anticancer activities of TLMs in vivo.
Figures


Similar articles
-
Functional characterization of tlmK unveiling unstable carbinolamide intermediates in the tallysomycin biosynthetic pathway.J Biol Chem. 2009 Mar 27;284(13):8256-64. doi: 10.1074/jbc.M900640200. Epub 2009 Feb 3. J Biol Chem. 2009. PMID: 19189972 Free PMC article.
-
The tallysomycin biosynthetic gene cluster from Streptoalloteichus hindustanus E465-94 ATCC 31158 unveiling new insights into the biosynthesis of the bleomycin family of antitumor antibiotics.Mol Biosyst. 2007 Jan;3(1):60-74. doi: 10.1039/b615284h. Epub 2006 Nov 28. Mol Biosyst. 2007. PMID: 17216057
-
Improved production of the tallysomycin H-1 in Streptoalloteichus hindustanus SB8005 strain by fermentation optimization.Appl Microbiol Biotechnol. 2010 May;86(5):1345-53. doi: 10.1007/s00253-009-2406-9. Epub 2010 Jan 5. Appl Microbiol Biotechnol. 2010. PMID: 20049429
-
Comparative analysis of the biosynthetic gene clusters and pathways for three structurally related antitumor antibiotics: bleomycin, tallysomycin, and zorbamycin.J Nat Prod. 2011 Mar 25;74(3):526-36. doi: 10.1021/np1008152. Epub 2011 Jan 6. J Nat Prod. 2011. PMID: 21210656 Free PMC article. Review.
-
The discovery and development of microbial bleomycin analogues.Appl Microbiol Biotechnol. 2018 Aug;102(16):6791-6798. doi: 10.1007/s00253-018-9129-8. Epub 2018 Jun 6. Appl Microbiol Biotechnol. 2018. PMID: 29876605 Review.
Cited by
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
-
Natural products for cancer chemotherapy.Microb Biotechnol. 2011 Nov;4(6):687-99. doi: 10.1111/j.1751-7915.2010.00221.x. Epub 2010 Nov 18. Microb Biotechnol. 2011. PMID: 21375717 Free PMC article. Review.
-
Complete genome sequence of the actinomycete Actinoalloteichus hymeniacidonis type strain HPA 177T isolated from a marine sponge.Stand Genomic Sci. 2016 Dec 20;11:91. doi: 10.1186/s40793-016-0213-3. eCollection 2016. Stand Genomic Sci. 2016. PMID: 28031775 Free PMC article.
-
A designer bleomycin with significantly improved DNA cleavage activity.J Am Chem Soc. 2012 Aug 15;134(32):13501-9. doi: 10.1021/ja3056535. Epub 2012 Aug 6. J Am Chem Soc. 2012. PMID: 22831455 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2009-2010.Mass Spectrom Rev. 2015 May-Jun;34(3):268-422. doi: 10.1002/mas.21411. Epub 2014 May 26. Mass Spectrom Rev. 2015. PMID: 24863367 Free PMC article. Review.
References
-
- Tao M, Wang L, Wendt-Pienkowski E, George NP, Galm U, Zhang G, Coughlin JM, Shen B. Mol. BioSyst. 2007;3:60–74. - PubMed
-
- Hecht SM. J. Nat. Prod. 2000;63:158–168. - PubMed
-
- Chen J, Stubbe J. Nat. Rev. Cancer. 2005;5:102–112. - PubMed
-
- Galm U, Hager MH, Van Lanen SG, Thorson JS, Shen B. Chem. Rev. 2005;106:739–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

