Therapy of hypoparathyroidism with intact parathyroid hormone
- PMID: 20094706
- PMCID: PMC2947814
- DOI: 10.1007/s00198-009-1149-x
Therapy of hypoparathyroidism with intact parathyroid hormone
Abstract
Hypoparathyroidism, a disorder characterized by low parathyroid hormone (PTH), is generally treated with oral calcium and vitamin D supplementation. We investigated the effects of PTH(1-84) treatment in 30 hypoparathyroid subjects for 24 months. PTH(1-84) treatment in hypoparathyroidism significantly reduced supplemental calcium and 1,25-dihydroxyvitamin D requirements without generally altering serum and urinary calcium levels.
Introduction: Hypoparathyroidism, a disorder characterized by low PTH, is associated with hypocalcemia, hypercalciuria, and increased bone mineral density (BMD). Conventional therapy with calcium and 1,25-dihydroxyvitamin D can maintain the serum calcium concentration, but doses are high, and control is variable. We investigated the effects of human PTH(1-84) treatment in hypoparathyroidism.
Methods: Thirty subjects with hypoparathyroidism were treated in an open-label study of PTH(1-84) 100 µg every other day by subcutaneous injection for 24 months, with monitoring of calcium and vitamin D supplementation requirements, serum and 24 h urinary calcium excretion, and BMD by dual energy X-ray absorptiometry.
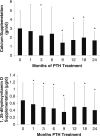
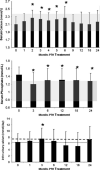
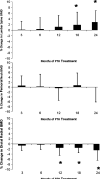
Results: Requirements for supplemental calcium decreased significantly (3,030±2,325 to 1,661±1,267 mg/day (mean±SD); p<0.05), as did requirements for supplemental 1,25-dihydroxyvitamin D (0.68±0.5 to 0.40±0.5 µg/day; p<0.05). Serum calcium levels and 24 h urinary calcium excretion were mostly unchanged at 24 months. BMD increased at the lumbar spine by 2.9±4% from baseline (p<0.05), while femoral neck BMD remained unchanged and distal one third radial BMD decreased by 2.4±4% (p<0.05).
Conclusion: PTH(1-84) treatment in hypoparathyroidism significantly reduces supplemental calcium and 1,25-dihydroxyvitamin D requirements without generally altering serum and urinary calcium levels.
Figures

References
-
- Marx S. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–1875. - PubMed
-
- Thakker R. Genetics of endocrine and metabolic disorders: parathyroid. Rev Endocr Metab Disord. 2004;5:37–51. - PubMed
-
- Rubin M, Levine M. Primer of metabolic bone diseases. American Society of Bone and Mineral Research; Washington: 2008. Hypoparathyroidism.
-
- Kao PC, van Heerden JA, Grant CS, Klee GG, Khosla S. Clinical performance of parathyroid hormone immunometric assays. Mayo Clin Proc. 1992;67:637–645. - PubMed
-
- Michelangeli VP, Heyma P, Colman PG, Ebeling PR. Evaluation of a new, rapid and automated immunochemilumino-metric assay for the measurement of serum intact parathyroid hormone. Ann Clin Biochem. 1997;34(Pt 1):97–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

