Tongue epithelial KT-1 cell-cycle arrest by TGF-beta associated with induction of p21(Cip1) and p15 (Ink4b)
- PMID: 20094776
- PMCID: PMC2825299
- DOI: 10.1007/s10616-010-9251-7
Tongue epithelial KT-1 cell-cycle arrest by TGF-beta associated with induction of p21(Cip1) and p15 (Ink4b)
Abstract
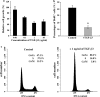
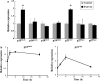
Tongue epithelium continuously turns over in adults. Our previous study showed that epidermal growth factor and fibroblast growth factor-2 stimulated proliferation of KT-1 cells derived from tongue epithelium, suggesting that these signals serve as positive regulators for tongue epithelial proliferation. To investigate a negative regulation of tongue epithelial cell proliferation, we studied effects of transforming growth factor-beta (TGF-beta) on KT-1 cells. Proliferation assays showed that TGF-beta inhibited proliferation of KT-1 cells in a dose dependent manner. Cell-cycle analysis showed that TGF-beta induced G(0)/G(1) cell cycle arrest in KT-1 cells. We also examined expressions of Ink4 and Cip/Kip family mRNA by quantitative reverse transcription-polymerase chain reaction. We found that TGF-beta induced p15(Ink4b) and p21(Cip1) mRNA expressions. These results strongly suggest that G(0)/G(1) cell cycle arrest is associated with increased p15(Ink4b) and p21(Cip1) expressions. Moreover, p21(Cip1) mRNA was localized in suprabasal cells of tongue epithelium, suggesting that p21(Cip1) play a role in cell-cycle exit along with tongue epithelial differentiation. Taken together, our results suggest that TGF-beta signaling serves as negative regulator of tongue epithelial cell proliferation, and may control tongue epithelial cell differentiation through modulating expression of p21(Cip1).
Figures


References
-
- Cheifetz S, Hernandez H, Laiho M, Ten Dijke P, Iwata KK, Massague J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990;265:20533–20538. - PubMed
-
- Ding QM, Ko TC, Evers BM. Caco-2 intestinal cell differentiation is associated with G1 arrest and suppression of CDK2 and CDK4. Am J Physiol. 1998;275:C1193–C1200. - PubMed
LinkOut - more resources
Full Text Sources

