Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial
- PMID: 20095068
- PMCID: PMC3645269
- DOI: 10.1097/ccm.0b013e3181c58715
Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial
Abstract
Objective: To demonstrate the feasibility of a placebo-controlled trial of antipsychotics for delirium in the intensive care unit and to test the hypothesis that antipsychotics would improve days alive without delirium or coma.
Design: Randomized, double-blind, placebo-controlled trial.
Setting: Six tertiary care medical centers in the US.
Patients: One hundred one mechanically ventilated medical and surgical intensive care unit patients.
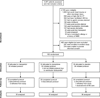
Intervention: Patients were randomly assigned to receive haloperidol or ziprasidone or placebo every 6 hrs for up to 14 days. Twice each day, frequency of study drug administration was adjusted according to delirium status, level of sedation, and side effects.
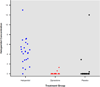
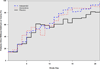
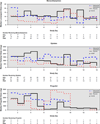
Measurements and main outcomes: The primary end point was the number of days patients were alive without delirium or coma. During the 21-day study period, patients in the haloperidol group spent a similar number days alive without delirium or coma (median [interquartile range], 14.0 [6.0-18.0] days) as did patients in the ziprasidone (15.0 [9.1-18.0] days) and placebo groups (12.5 [1.2-17.2] days; p = 0.66). No differences were found in secondary clinical outcomes, including ventilator-free days (p = .25), hospital length of stay (p = .68), and mortality (p = .81). Ten (29%) patients in the haloperidol group reported symptoms consistent with akathisia, compared with six (20%) patients in the ziprasidone group and seven (19%) patients in the placebo group (p = .60), and a global measure of extrapyramidal symptoms was similar between treatment groups (p = .46).
Conclusions: A randomized, placebo-controlled trial of antipsychotics for delirium in mechanically ventilated intensive care unit patients is feasible. Treatment with antipsychotics in this limited pilot trial did not improve the number of days alive without delirium or coma, nor did it increase adverse outcomes. Thus, a large trial is needed to determine whether use of antipsychotics for intensive care unit delirium is appropriate.
Trial registration: ClinicalTrials.gov NCT00096863.
Figures
Comment in
-
Free your MIND and the rest will follow: decoding delirium in the intensive care unit.Crit Care Med. 2010 Feb;38(2):697-8. doi: 10.1097/CCM.0b013e3181c89542. Crit Care Med. 2010. PMID: 20083930 No abstract available.
-
Clearing up the confusion: the results of two pilot studies of antipsychotics for ICU delirium.Crit Care. 2010;14(4):316. doi: 10.1186/cc9200. Epub 2010 Aug 11. Crit Care. 2010. PMID: 20735864 Free PMC article. No abstract available.
References
-
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. - PubMed
-
- Micek ST, Anand NJ, Laible BR, et al. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33:1260–1265. - PubMed
-
- Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

