Crystallographic characterization of N-oxide tripod amphiphiles
- PMID: 20095541
- PMCID: PMC3090072
- DOI: 10.1021/ja9085148
Crystallographic characterization of N-oxide tripod amphiphiles
Erratum in
- J Am Chem Soc. 2013 Aug 21;135(33):12477
Abstract
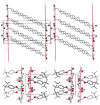
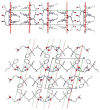
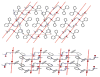
Tripod amphiphiles are designed to promote the solubilization and stabilization of intrinsic membrane proteins in aqueous solution; facilitation of crystallization is a long-range goal. Membrane proteins are subjects of extensive interest because of their critical biological roles, but proteins of this type can be difficult to study because of their low solubility in water. The nonionic detergents that are typically used to achieve solubility can have the unintended effect of causing protein denaturation. Tripod amphiphiles differ from conventional detergents in that the lipophilic segment contains a branchpoint, and previous work has shown that this unusual amphiphilic architecture can be advantageous relative to traditional detergent structures. Here, we report the crystal structures of several tripod amphiphiles that contain an N-oxide hydrophilic group. The data suggest that tripods can adapt themselves to a nonpolar surface by altering the hydrophobic appendage that projects toward that surface and their overall orientation relative to that surface. Although it is not possible to draw firm conclusions regarding amphiphile association in solution from crystallographic data, trends observed among the packing patterns reported here suggest design strategies to be implemented in future studies.
Figures

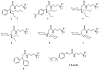

Similar articles
-
Novel tripod amphiphiles for membrane protein analysis.Chemistry. 2013 Nov 11;19(46):15645-51. doi: 10.1002/chem.201301423. Epub 2013 Oct 2. Chemistry. 2013. PMID: 24123610 Free PMC article.
-
Hydrophobic variations of N-oxide amphiphiles for membrane protein manipulation: importance of non-hydrocarbon groups in the hydrophobic portion.Chem Asian J. 2014 Jan;9(1):110-6. doi: 10.1002/asia.201301097. Epub 2013 Nov 4. Chem Asian J. 2014. PMID: 24347070 Free PMC article.
-
An improved tripod amphiphile for membrane protein solubilization.Protein Sci. 2000 Dec;9(12):2518-27. doi: 10.1110/ps.9.12.2518. Protein Sci. 2000. PMID: 11206073 Free PMC article.
-
Successful amphiphiles as the key to crystallization of membrane proteins: Bridging theory and practice.Biochim Biophys Acta Gen Subj. 2019 Feb;1863(2):437-455. doi: 10.1016/j.bbagen.2018.11.004. Epub 2018 Nov 10. Biochim Biophys Acta Gen Subj. 2019. PMID: 30419284 Review.
-
The mechanism of detergent solubilization of lipid bilayers.Biophys J. 2013 Jul 16;105(2):289-99. doi: 10.1016/j.bpj.2013.06.007. Biophys J. 2013. PMID: 23870250 Free PMC article. Review.
Cited by
-
A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins.Chemistry. 2012 Jul 27;18(31):9485-90. doi: 10.1002/chem.201200069. Epub 2012 Jun 22. Chemistry. 2012. PMID: 22730191 Free PMC article. No abstract available.
-
Mechanistic analysis of ghrelin-O-acyltransferase using substrate analogs.Bioorg Chem. 2015 Oct;62:64-73. doi: 10.1016/j.bioorg.2015.07.003. Epub 2015 Jul 13. Bioorg Chem. 2015. PMID: 26246082 Free PMC article.
-
Hydrophobic variants of ganglio-tripod amphiphiles for membrane protein manipulation.Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):278-86. doi: 10.1016/j.bbamem.2013.09.011. Epub 2013 Sep 21. Biochim Biophys Acta. 2014. PMID: 24064323 Free PMC article.
-
Synthesis and properties of dodecyl trehaloside detergents for membrane protein studies.Langmuir. 2012 Jul 31;28(30):11173-81. doi: 10.1021/la3020404. Epub 2012 Jul 20. Langmuir. 2012. PMID: 22780816 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

