Synthesis of 7-(15)N-Oroidin and evaluation of utility for biosynthetic studies of pyrrole-imidazole alkaloids by microscale (1)H-(15)N HSQC and FTMS
- PMID: 20095632
- PMCID: PMC3544367
- DOI: 10.1021/np900638e
Synthesis of 7-(15)N-Oroidin and evaluation of utility for biosynthetic studies of pyrrole-imidazole alkaloids by microscale (1)H-(15)N HSQC and FTMS
Abstract
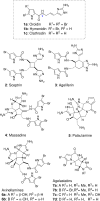
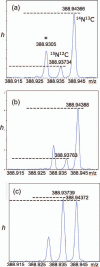
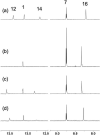
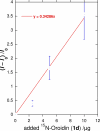
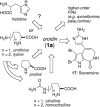
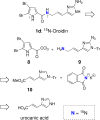
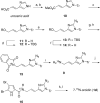
Numerous marine-derived pyrrole-imidazole alkaloids (PIAs), ostensibly derived from the simple precursor oroidin, 1a, have been reported and have garnered intense synthetic interest due to their complex structures and in some cases biological activity; however very little is known regarding their biosynthesis. We describe a concise synthesis of 7-(15)N-oroidin (1d) from urocanic acid and a direct method for measurement of (15)N incorporation by pulse labeling and analysis by 1D (1)H-(15)N HSQC NMR and FTMS. Using a mock pulse labeling experiment, we estimate the limit of detection (LOD) for incorporation of newly biosynthesized PIA by 1D (1)H-(15)N HSQC to be 0.96 microg equivalent of (15)N-oroidin (2.4 nmole) in a background of 1500 microg of unlabeled oroidin (about 1 part per 1600). 7-(15)N-Oroidin will find utility in biosynthetic feeding experiments with live sponges to provide direct information to clarify the pathways leading to more complex pyrrole-imidazole alkaloids.
Figures


Similar articles
-
Pyrrole carboxamide introduction in the total synthesis of pyrrole-imidazole alkaloids.Org Biomol Chem. 2021 Mar 28;19(12):2603-2621. doi: 10.1039/d0ob02052d. Epub 2021 Mar 8. Org Biomol Chem. 2021. PMID: 33683231 Review.
-
A submarine journey: the pyrrole-imidazole alkaloids.Mar Drugs. 2009 Nov 27;7(4):705-53. doi: 10.3390/md7040705. Mar Drugs. 2009. PMID: 20098608 Free PMC article. Review.
-
De novo synthesis of benzosceptrin C and nagelamide H from 7-15N-oroidin: implications for pyrrole-aminoimidazole alkaloid biosynthesis.J Nat Prod. 2012 Apr 27;75(4):527-30. doi: 10.1021/np300051k. Epub 2012 Mar 28. J Nat Prod. 2012. PMID: 22455452 Free PMC article.
-
Diversity-oriented approach to pyrrole-imidazole alkaloid frameworks.Org Lett. 2011 Mar 18;13(6):1382-5. doi: 10.1021/ol200067e. Epub 2011 Feb 21. Org Lett. 2011. PMID: 21338082 Free PMC article.
-
Discovery of Anti-Inflammatory Alkaloids from Sponge Stylissa massa Suggests New Biosynthetic Pathways for Pyrrole-Imidazole Alkaloids.Mar Drugs. 2024 Oct 18;22(10):477. doi: 10.3390/md22100477. Mar Drugs. 2024. PMID: 39452885 Free PMC article.
Cited by
-
Syntheses of Cyclic Guanidine-Containing Natural Products.Tetrahedron. 2015 Feb 25;71(8):1145-1173. doi: 10.1016/j.tet.2014.11.056. Tetrahedron. 2015. PMID: 25684829 Free PMC article.
-
Asymmetric total synthesis of ent-cyclooroidin.Org Lett. 2010 Nov 5;12(21):4940-3. doi: 10.1021/ol1020916. Org Lett. 2010. PMID: 20929213 Free PMC article.
-
Dimeric pyrrole-imidazole alkaloids: synthetic approaches and biosynthetic hypotheses.Chem Commun (Camb). 2014 Aug 14;50(63):8628-39. doi: 10.1039/c4cc02290d. Epub 2014 May 15. Chem Commun (Camb). 2014. PMID: 24828265 Free PMC article. Review.
-
Structures and solution conformational dynamics of stylissamides G and H from the Bahamian sponge Stylissa caribica.J Nat Prod. 2014 Mar 28;77(3):625-30. doi: 10.1021/np400891s. Epub 2014 Feb 27. J Nat Prod. 2014. PMID: 24576291 Free PMC article.
-
Bioinspired total synthesis of agelastatin A.Angew Chem Int Ed Engl. 2012 Jul 9;51(28):6870-3. doi: 10.1002/anie.201200959. Epub 2012 Jun 11. Angew Chem Int Ed Engl. 2012. PMID: 22689447 Free PMC article.
References
-
-
Recent examples of syntheses of pyrrole-imidazole alkaloids: Arndt H-D, Koert U. In: Organic Synthesis Highlights 4. Schmalz H-G, editor. Wiley-VCH; Weinheim: 2000. p. 241.; Hoffmann H, Lindel T. Synthesis. 2003:1753–1783.; Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551–1565.; Du H, He Y, Sivappa R, Lovely C. J. Synlett. 2006:965–992.; Kock M, Grube A, Seiple IB, Baran PS. Angew. Chem. 2007;119:6706–6714.; Angew. Chem. Int. Ed. 2007;46:6586–6594.; Weinreb SM. Nat. Prod. Rep. 2007;24:931–948.; For some publications not covered in the these reviews, see: Dransfield PJ, Dilley AS, Wang S, Romo D. Tetrahedron. 2006;62:5223–5247.; Wang S, Dilley AS, Poullennec KG, Romo D. Tetrahedron. 2006;62:7155–7161.; Nakadai M, Harran PG. Tetrahedron Lett. 2006;47:3933–3935.; Tan X, Chen C. Angew. Chem., Int. Ed. 2006;45:4345–4348.; Overman LE, Paulini R, White NS. J. Am. Chem. Soc. 2007;129:12896–2900.; O'Malley D, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2008;47:3581–3583. ; Yamaguchi J, Seiple IB, Young IS, O'Malley DP, Maue M, Baran PS. Angew. Chem. Int. Ed. 2008;47:3578–3580.; Bhandari MR, Sivappa R, Lovely CJ. Org. Lett. 2009;11:1535–1538.
-
-
- Al Mourabit A, Potier P. Eur. J. Org. Chem. 2001:237–243.
-
-
Walker RP, Faulkner DJ, VanEngen D, Clardy J. J. Am. Chem. Soc. 1981;103:6772–6773. An variation on the sceptrin skeleton, embodying an unusual benzocyclobutane (benzosceptrins A and B), has just been reported. Appenzeller J, Tilvi S, Martin M-T, Gallard J-F, El-bitar H, Dau ETH, Debitus C, Laurent D, Moriou C, Al-Mourabit A. Org. Lett. 2009;11:4874–4877.
-
-
- Keifer PA, Koker MES, Schwartz RE, Hughes RG, Jr., Rittschof D, Rinehart KL. 26th Interscience Conference on Antimicrobial Agents and Chemotherapy; New Orleans. Sep. 28–Oct. 1, 1986; No. 1281.
- Rinehart KL. Pure Appl. Chem. 1989;6:525–528.
- Kobayashi J, Tsuda M, Murayama T, Nakamura H, Ohizumi Y, Ishibashi M, Iwamura M, Ohta T, Nozoe S. Tetrahedron. 1990;46:5579–5586.
- Keifer PA, Schwartz RE, Koker MES, Hughes RG, Rittschof D, Rinehart KL. J. Org. Chem. 1991;56:2965–2975.
- Williams DH, Faulkner DJ. Tetrahedron. 1996;52:5381–5390.
-
- Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, van Soest RWM, Fusetani N. Org. Lett. 2003;5:2255–2257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

