Design of polyamine-based therapeutic agents: new targets and new directions
- PMID: 20095971
- PMCID: PMC3564240
- DOI: 10.1042/bse0460006
Design of polyamine-based therapeutic agents: new targets and new directions
Abstract
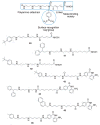
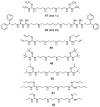
Enzymes in the biosynthetic and catabolic polyamine pathway have long been considered targets for drug development, and early drug discovery efforts in the polyamine area focused on the design and development of specific inhibitors of the biosynthetic pathway, or polyamine analogues that specifically bind DNA. More recently, it has become clear that the natural polyamines are involved in numerous known and unknown cellular processes, and disruption of polyamine functions at their effector sites can potentially produce beneficial therapeutic effects. As new targets for polyamine drug discovery continue to evolve, the rational design of polyamine analogues will result in more structurally diverse agents. In addition, the physical linkage of polyamine-like structures to putative drug molecules can have beneficial effects resulting from increases in DNA affinity and selective cellular uptake. The present chapter will summarize recent advances in the development of alkylpolyamine analogues as antitumour agents, and describe subsequent advances that have resulted from incorporating polyamine character into more diverse drug molecules. Specifically, new polyamine analogues, and the role of polyamine fragments in the design of antiparasitic agents, antitumour metal complexes, histone deacetylase inhibitors and lysine-specific demethylase 1 inhibitors, will be described.
Figures




Similar articles
-
Polyamine analogues targeting epigenetic gene regulation.Essays Biochem. 2009 Nov 4;46:95-110. doi: 10.1042/bse0460007. Essays Biochem. 2009. PMID: 20095972 Free PMC article. Review.
-
Selective regulation of polyamine metabolism with methylated polyamine analogues.Amino Acids. 2014 Mar;46(3):605-20. doi: 10.1007/s00726-013-1587-9. Epub 2013 Oct 10. Amino Acids. 2014. PMID: 24022706 Review.
-
Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues.Proc West Pharmacol Soc. 2005;48:24-30. Proc West Pharmacol Soc. 2005. PMID: 16416654 Review.
-
Development of polyamine analogs as cancer therapeutic agents.Oncol Res. 2002;13(3):123-35. Oncol Res. 2002. PMID: 12555742 Review.
-
Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 2. Structural analogues and derivatives.Curr Drug Targets. 2003 Oct;4(7):565-85. doi: 10.2174/1389450033490876. Curr Drug Targets. 2003. PMID: 14535655 Review.
Cited by
-
Current status of the polyamine research field.Methods Mol Biol. 2011;720:3-35. doi: 10.1007/978-1-61779-034-8_1. Methods Mol Biol. 2011. PMID: 21318864 Free PMC article. Review.
-
Silica nanoparticle remodeling under mild conditions: versatile one step conversion of mesoporous to hollow nanoparticles with simultaneous payload loading.Nanoscale. 2022 Dec 8;14(47):17514-17518. doi: 10.1039/d2nr05528g. Nanoscale. 2022. PMID: 36408868 Free PMC article.
-
Identification of Deregulated Signaling Pathways in Jurkat Cells in Response to a Novel Acylspermidine Analogue-N4-Erucoyl Spermidine.Epigenet Insights. 2018 Nov 27;11:2516865718814543. doi: 10.1177/2516865718814543. eCollection 2018. Epigenet Insights. 2018. PMID: 30515476 Free PMC article.
-
Polyamines in aging and disease.Aging (Albany NY). 2011 Aug;3(8):716-32. doi: 10.18632/aging.100361. Aging (Albany NY). 2011. PMID: 21869457 Free PMC article. Review.
-
Purvalanol A is a strong apoptotic inducer via activating polyamine catabolic pathway in MCF-7 estrogen receptor positive breast cancer cells.Mol Biol Rep. 2014 Jan;41(1):145-54. doi: 10.1007/s11033-013-2847-1. Epub 2013 Nov 5. Mol Biol Rep. 2014. PMID: 24190492
References
-
- Casero RA, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. - PubMed
-
- Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. - PubMed
-
- Bey P, Danzin C, Van Dorsselaer V, Mamont P, Jung M, Tardif C. Analogues of ornithine as inhibitors of ornithine decarboxylase. New deductions concerning the topography of the enzyme’s active site. J Med Chem. 1978;21:50–55. - PubMed
-
- Mamont PS, Duchesne MC, Grove J, Bey P. Anti-proliferative properties of DL-α-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81:58–66. - PubMed
-
- Levin VA, Hess KR, Choucair A, Flynn PJ, Jaeckle KA, Kyritsis AP, Yung WK, Prados MD, Bruner JM, Ictech S, et al. Phase III randomized study of postradiotherapy chemotherapy with combination α-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

