Plasminogen on the surfaces of fibrin clots prevents adhesion of leukocytes and platelets
- PMID: 20095998
- PMCID: PMC4530790
- DOI: 10.1111/j.1538-7836.2010.03778.x
Plasminogen on the surfaces of fibrin clots prevents adhesion of leukocytes and platelets
Abstract
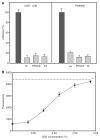
Background and objectives: Although leukocytes and platelets adhere to fibrin with alacrity in vitro, these cells do not readily accumulate on the surfaces of fibrin clots in vivo. The difference in the capacity of blood cell integrins to adhere to fibrin in vivo and in vitro is striking and implies the existence of a physiologic antiadhesive mechanism. The surfaces of fibrin clots in the circulation are continually exposed to plasma proteins, several of which can bind fibrin and influence cell adhesion. Recently, we have demonstrated that adsorption of soluble fibrinogen on the surface of a fibrin clot results in its deposition as a soft multilayer matrix, which prevents attachment of blood cells. In the present study, we demonstrate that another plasma protein, plasminogen, which is known to accumulate in the superficial layer of fibrin, exerts an antiadhesive effect.
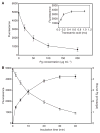
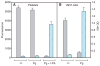
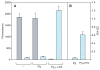
Results: After being coated with plasminogen, the surfaces of fibrin clots became essentially non-adhesive for U937 monocytic cells, blood monocytes, and platelets. The data revealed that activation of fibrin-bound plasminogen by the plasminogen-activating system assembled on adherent cells resulted in the generation of plasmin, which decomposed the superficial fibrin layer, resulting in cell detachment under flow. The surfaces generated after the initial cell adhesion remained non-adhesive for subsequent attachment of leukocytes and platelets.
Conclusion: We propose that the limited degradation of fibrin by plasmin generated by adherent cells loosens the fibers on the clot surface, producing a mechanically unstable substrate that is unable to support firm integrin-mediated cell adhesion.
Conflict of interest statement
This work was supported by the National Institutes of Health.
Figures


Similar articles
-
Fibrinogen counteracts the antiadhesive effect of fibrin-bound plasminogen by preventing its activation by adherent U937 monocytic cells.J Thromb Haemost. 2012 Jun;10(6):1081-90. doi: 10.1111/j.1538-7836.2012.04745.x. J Thromb Haemost. 2012. PMID: 22507544 Free PMC article.
-
Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner.J Trauma Acute Care Surg. 2019 Jan;86(1):101-107. doi: 10.1097/TA.0000000000002092. J Trauma Acute Care Surg. 2019. PMID: 30575685
-
Control of integrin alphaIIb beta3 outside-in signaling and platelet adhesion by sensing the physical properties of fibrin(ogen) substrates.Biochemistry. 2010 Jan 12;49(1):68-77. doi: 10.1021/bi9016022. Biochemistry. 2010. PMID: 19929007 Free PMC article.
-
Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications.Int J Mol Sci. 2022 May 28;23(11):6065. doi: 10.3390/ijms23116065. Int J Mol Sci. 2022. PMID: 35682744 Free PMC article. Review.
-
The hemostatic system.Curr Med Chem. 2004 Sep;11(17):2245-60. doi: 10.2174/0929867043364603. Curr Med Chem. 2004. PMID: 15379710 Review.
Cited by
-
Fibrinogen counteracts the antiadhesive effect of fibrin-bound plasminogen by preventing its activation by adherent U937 monocytic cells.J Thromb Haemost. 2012 Jun;10(6):1081-90. doi: 10.1111/j.1538-7836.2012.04745.x. J Thromb Haemost. 2012. PMID: 22507544 Free PMC article.
-
Whole-genome sequencing and gene network modules predict gemcitabine/carboplatin-induced myelosuppression in non-small cell lung cancer patients.NPJ Syst Biol Appl. 2020 Aug 24;6(1):25. doi: 10.1038/s41540-020-00146-6. NPJ Syst Biol Appl. 2020. PMID: 32839457 Free PMC article.
-
Study on the activity of recombinant mutant tissue-type plasminogen activator fused with the C-terminal fragment of hirudin.J Thromb Thrombolysis. 2021 Oct;52(3):880-888. doi: 10.1007/s11239-021-02440-4. Epub 2021 Apr 7. J Thromb Thrombolysis. 2021. PMID: 33826053
-
Synthetic hydrogels as blood clot mimicking wound healing materials.Prog Biomed Eng (Bristol). 2021 Oct;3(4):042006. doi: 10.1088/2516-1091/ac23a4. Epub 2021 Sep 30. Prog Biomed Eng (Bristol). 2021. PMID: 35822083 Free PMC article.
-
The assembly of nonadhesive fibrinogen matrices depends on the αC regions of the fibrinogen molecule.J Biol Chem. 2012 Dec 7;287(50):41979-90. doi: 10.1074/jbc.M112.410696. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086938 Free PMC article.
References
-
- Kuijper PHM, Torres HIG, van der Linden JAM, Lammers JWJ, Sixma JJ, Zwaginga JJ, Koenderman L. Neutrophil adhesion to fibrinogen and fibrin under flow conditions is diminished by activation and L-selectin shedding. Blood. 1997;89:2131–8. - PubMed
-
- Kuijper PHM, Torres G, Lammers J-WJ, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood. 1997;89:166–75. - PubMed
-
- Hantgan RR, Endenburg SC, Cavero I, Marguerie G, Uzan A, Sixma JJ, de Groot PG. Inhibition of platelet adhesion to fibrin(ogen) in flowing whole blood by arg–gly–asp and fibrinogen gamma-chain carboxy terminal peptides. Thromb Haemost. 1992;68:694–700. - PubMed
-
- Arfors K-E, Dhall DP, Engeset J, Hint H, Matherson NA, Tangen O. Biolaser endothelial trauma as a means of quantifying platelet activity in vivo. Nature. 1968;218:887–8.
-
- Baumgartner HR. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973;5:167–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

