Serial bone marrow transplantation reveals in vivo expression of the pCLPG retroviral vector
- PMID: 20096105
- PMCID: PMC2845565
- DOI: 10.1186/1743-422X-7-16
Serial bone marrow transplantation reveals in vivo expression of the pCLPG retroviral vector
Abstract
Background: Gene therapy in the hematopoietic system remains promising, though certain aspects of vector design, such as transcriptional control elements, continue to be studied. Our group has developed a retroviral vector where transgene expression is controlled by p53 with the intention of harnessing the dynamic and inducible nature of this tumor suppressor and transcription factor. We present here a test of in vivo expression provided by the p53-responsive vector, pCLPG. For this, we used a model of serial transplantation of transduced bone marrow cells.
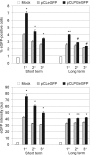
Results: We observed, by flow cytometry, that the eGFP transgene was expressed at higher levels when the pCLPG vector was used as compared to the parental pCL retrovirus, where expression is directed by the native MoMLV LTR. Expression from the pCLPG vector was longer lasting, but did decay along with each sequential transplant. The detection of eGFP-positive cells containing either vector was successful only in the bone marrow compartment and was not observed in peripheral blood, spleen or thymus.
Conclusions: These findings indicate that the p53-responsive pCLPG retrovirus did offer expression in vivo and at a level that surpassed the non-modified, parental pCL vector. Our results indicate that the pCLPG platform may provide some advantages when applied in the hematopoietic system.
Figures


Similar articles
-
pCLPG: a p53-driven retroviral system.Virology. 2004 Apr 10;321(2):165-72. doi: 10.1016/j.virol.2003.12.021. Virology. 2004. PMID: 15051377
-
Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector.Blood. 1999 Nov 15;94(10):3349-57. Blood. 1999. PMID: 10552944 Free PMC article.
-
Transgene expression levels determine the immunogenicity of transduced hematopoietic grafts in partially myeloablated mice.Mol Ther. 2009 Nov;17(11):1904-9. doi: 10.1038/mt.2009.198. Epub 2009 Aug 25. Mol Ther. 2009. PMID: 19707185 Free PMC article.
-
Activation of endogenous p53 by combined p19Arf gene transfer and nutlin-3 drug treatment modalities in the murine cell lines B16 and C6.BMC Cancer. 2010 Jun 22;10:316. doi: 10.1186/1471-2407-10-316. BMC Cancer. 2010. PMID: 20569441 Free PMC article.
-
Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector.Blood. 2003 Jan 15;101(2):485-91. doi: 10.1182/blood-2002-07-2131. Epub 2002 Sep 5. Blood. 2003. PMID: 12393475
References
-
- Nair V. Retrovirus-induced oncogenesis and safety of retroviral vectors. Curr Opin Mol Ther. 2008;10:431–438. - PubMed
-
- Hacein-Bey H, Cavazzana-Calvo M, Le Deist F, Dautry-Varsat A, Hivroz C, Riviere I, Danos O, Heard JM, Sugamura K, Fischer A, De Saint Basile G. gamma-c gene transfer into SCID X1 patients' B-cell lines restores normal high-affinity interleukin-2 receptor expression and function. Blood. 1996;87:3108–3116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

